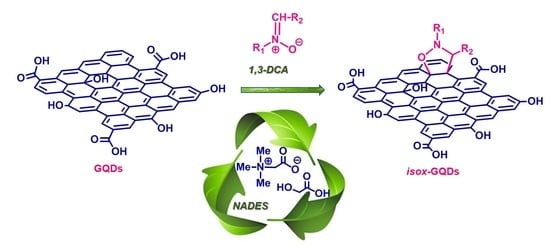
Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical, Physical, and Morphological Characterization
2.3. Synthesis of GQDs
2.4. Synthesis of NADES
2.5. Synthesis of C-(diethoxyphosphoryl)propylidene, N-benzyl nitrone 1b
2.6. Synthesis of isox-GQDs 2a and isox-GQDs 2b
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, 1904362. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, 1902136. [Google Scholar] [CrossRef]
- Iannazzo, D.; Ziccarelli, I.; Pistone, A. Graphene quantum dots: Multifunctional nanoplatforms for anticancer therapy. J. Mater. Chem. B 2017, 5, 6471–6489. [Google Scholar] [CrossRef]
- Iannazzo, D.; Celesti, C.; Espro, C. Recent Advances on Graphene Quantum Dots as Multifunctional Nanoplatforms for Cancer Treatment. Biotechnol. J. 2020, 1900422. [Google Scholar] [CrossRef]
- Tade, R.S.; Patil, P.O. Theranostic Prospects of Graphene Quantum Dots in Breast Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5987–6008. [Google Scholar]
- Fan, X.; Phebus, B.D.; Li, L.; Chen, S. Chemical Functionalization of Graphene Quantum Dots. Sci. Adv. Mater. 2015, 7, 1990–2010. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Galvagno, S. Functionalization methods of graphene. In Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications. Thakur, V.K., Thakur, M.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 510–537. [Google Scholar]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patané, S.; Giofré, S.V.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Iannazzo, D.; Pistone, A.; Ferro, S.; De Luca, L.; Monforte, A.M.; Romeo, R.; Buemi, M.R.; Pannecouque, C. Graphene Quantum Dots Based Systems As HIV Inhibitors. Bioconjugate Chem. 2018, 29, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3- Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, G.; Scala, A.; Fazio, E.; Mineo, P.G.; Rescifina, A.; Piperno, A.; Grassi, G. Repurposing of oxazolone chemistry: Gaining access to functionalized graphene nanosheets in a top-down approach from graphite. Chem. Sci. 2015, 6, 6961–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Yu, W.; Xiao, Z.; Song, Y.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, C. A 1,3-dipolar cycloaddition protocol to porphyrin-functionalized reduced graphene oxide with a push-pull motif. Nano Res. 2015, 8, 870–886. [Google Scholar] [CrossRef]
- Tagmatarchis, N.; Prato, M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J. Mater. Chem. 2004, 14, 437–439. [Google Scholar] [CrossRef]
- Ghini, G.; Luconi, L.; Rossin, A.; Bianchini, C.; Giambastiani, G.; Cicchi, S.; Lascialfari, L.; Brandi, A.; Giannasi, A. Can nitrones functionalize carbon nanotubes? Chem. Commun. 2010, 46, 252–254. [Google Scholar] [CrossRef]
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Commun. 2012, 48, 6836–6838. [Google Scholar] [CrossRef]
- Prato, M.; Suzuki, T.; Foroudian, H.; Li, Q.; Khemani, K.; Wudl, F.; Leonetti, J.; Little, R.D.; White, T.; Yamago, S.; et al. [3 + 2] and [4 + 2] Cycloadditions of fullerene C60. J. Am. Chem. Soc. 1993, 115, 1594–1595. [Google Scholar] [CrossRef]
- Akhmetov, A.R.; Tuktarov, A.R.; Popod’ko, N.R.; Dzhemilev, U.M. Cycloaddition of alkyl azides to fullerene C60 in the presence of Cu(OTf)2. Mendeleev Commun. 2015, 25, 346–347. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef]
- Sekiya, R.; Uemura, Y.; Murakami, H.; Haino, T. White-Light-Emitting Edge-Functionalized Graphene Quantum Dots. Angew. Chem. 2014, 126, 5725–5729. [Google Scholar] [CrossRef]
- Qi, B.-P.; Hu, H.; Bao, L.; Zhang, Z.-L.; Tang, B.; Peng, Y.; Wang, B.-S.; Pang, D.-W. An efficient edge-functionalization method to tune the photoluminescence of graphene quantum dots. Nanoscale 2015, 7, 5969–5973. [Google Scholar] [CrossRef] [PubMed]
- Tiecco, M.; Cappellini, F.; Nicoletti, F.; Del Giacco, T.; Germani, R.; Di Profio, P. Role of the hydrogen bond donor component for a proper development of novel hydrophobic deep eutectic solvents. J. Mol. Liq. 2019, 281, 423–430. [Google Scholar] [CrossRef]
- Lu, J.; Li, W.-T.; Ma, E.-Q.; Mo, L.-P.; Zhang, Z.-H. Inside Back Cover: Superparamagnetic CuFeO2 Nanoparticles in Deep Eutectic Solvent: An Efficient and Recyclable Catalytic System for the Synthesis of Imidazo[1,2-α]pyridines. ChemCatChem 2014, 6, 2854–2859. [Google Scholar] [CrossRef]
- Müller, C.R.; Meiners, I.; de Domínguez María, P. Highly enantioselective tandem enzyme–organocatalyst crossed aldol reactions with acetaldehyde in deep-eutectic-solvents. RSC Adv. 2014, 4, 46097–46101. [Google Scholar]
- Perez, J.M.; Ramòn, D.J. Synthesis of 3,5-disubstituted isoxazoles and isoxazolines in deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 2343–2349. [Google Scholar] [CrossRef] [Green Version]
- Pinto Martins, M.A.; Caneppele Paveglio, G.; Valvassori Rodrigues, L.; Piccinin Frizzo, C.; Zanatta, N.; Gauze Bonacorso, H. Promotion of 1,3-dipolar cycloaddition between azides and β-enaminones by deep eutectic solvents. New J. Chem. 2016, 40, 5989–5992. [Google Scholar] [CrossRef]
- Curti, F.; Tiecco, M.; Pirovano, V.; Germani, R.; Caselli, A.; Rossi, E.; Abbiati, G. p-TSA-Based DESs as “Active Green Solvents” for Microwave Enhanced Cyclization of 2-Alkynyl-(hetero)-arylcarboxylates: An Alternative Access to 6-Substituted 3,4-Fused 2-Pyranones. Eur. J. Org. Chem. 2019, 9, 1904–1914. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Requejo, P.F.; Kroon, M.C. Aliphatic−Aromatic Separation Using Deep Eutectic Solvents as Extracting Agents. Ind. Eng. Chem. Res. 2015, 54, 11404–11412. [Google Scholar] [CrossRef]
- Iannazzo, D.; Brunaccini, E.; Giofrè, S.V.; Piperno, A.; Romeo, G.; Ronsisvalle, S.; Chiacchio, M.A.; Lanza, G.; Chiacchio, U. Competitive Formation of Enaminones and 3-Amino-2(5H)-furanones from the Isoxazolidine System: A Combined Synthetic and Quantum Chemical Study. Eur. J. Org. Chem. 2010, 5897–5905. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Tiecco, M.; Zappacosta, R.; Boncompagni, S.; Di Profio, P.; Ettorre, V.; Fontana, A.; Germani, R.; Siani, G. Novel zwitterionic Natural Deep Eutectic Solvents as environmentally friendly media for spontaneous self-assembly of gold nanoparticles. J. Mol. Liq. 2018, 268, 371–375. [Google Scholar] [CrossRef]
- Donato, M.G.; Galvagno, S.; Messina, G.; Milone, C.; Pistone, A.; Santangelo, S. Optimisation of gas mixture composition for the preparation of high quality MWCNT by catalytically assisted CVD. Diam. Relat. Mater. 2007, 16, 1095–1100. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S.; Romeo, R.; Giofré, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Gulino, A. Structural and electronic characterization of self-assembled molecular nanoarchitectures by X-rayphotoelectron spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; IM Publications: Chichester, UK; Surface Spectra Ltd.: Manchester, UK, 2003. [Google Scholar]
- Cardellini, F.; Tiecco, M.; Germani, R.; Cardinali, G.; Corte, L.; Roscini, L.; Spreti, N. Novel zwitterionic deep eutectic solvents from trimethylglycine and carboxylic acids: Characterization of their properties and their toxicity. RSC Adv. 2014, 4, 55990–56002. [Google Scholar] [CrossRef]
- Lavin, J.G.; Subramoney, S.; Ruoff, R.; Berber, S.; Tomanek, D. Scrolls and nested tubes in multiwall carbon nanotubes. Carbon 2002, 40, 1123–1130. [Google Scholar] [CrossRef]
- Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Chiacchio, U.; Rescifina, A.; Balestrieri, E.; Macchi, B.; Mastino, A.; Cortese, R. 3-Amino-2(5H)furanones as inhibitors of subgenomic hepatitis C virus RNA replication. Bioorg. Med. Chem. 2008, 16, 9610–9615. [Google Scholar] [CrossRef]
- Camper, N.; Scott, C.J.; Migaud, M. Synthesis of an analogue of the bisphosphonate drug Ibandronate for targeted drug-delivery therapeutic strategies. New J. Chem. 2010, 34, 949–955. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Afshari, R.; Ramón, D.J.; Varma, R.S. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020, 22, 3668–3692. [Google Scholar]
- Uceta, H.; Vizuete, M.; Carrillo, J.R.; Barrejón, M.; Fierro, G.J.L.; Prieto, M.P.; Langa, F. Cycloaddition of Nitrile Oxides to Graphene: A Theoretical and Experimental Approach. Chem. Eur. J. 2019, 25, 14644–14650. [Google Scholar] [CrossRef]
- Ferrándiz-Saperas, M.; Ghisolfi, A.; Cazorla-Amorós, D.; Nájera, C.; Sansano, J.M. Multilayer graphene functionalized through thermal 1,3-dipolar cycloadditions with imino esters: A versatile platform for supported ligands in catalysis. Chem. Commun. 2019, 55, 7462–7465. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, J.; Kaminker, R.; Motiei, L.; de Ruiter, G.; Morozov, M.; Lupo, F.; Gulino, A.; Van der Boom, M.E. Linear vs Exponential Formation of Molecular-Based Assemblies. J. Am. Chem. Soc. 2010, 132, 9295–9297. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Han, J.; Yang, C.T.; Cheng, C.M.; Luo, Y.M.; Wang, X.L. A category of hierarchically porous tin (IV) phosphonate backbone with the implication for radioanalytical separation. Chem. Eng. J. 2016, 302, 368–376. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Malviya, R.; Sharma, P.K. Measurement Techniques and Pharmaceutical Applications of Zeta Potential: A Review. J. Drug Deliv. Ther. 2014, 4, 33–40. [Google Scholar]
- Huang, C.L.; Huang, C.C.; Mai, F.D.; Yen, C.L.; Tzing, S.H.; Hsieh, H.T.; Ling, Y.C.; Chang, J.Y. Application of paramagnetic graphene quantum dots as a platform for simultaneous dual-modality bioimaging and tumor-targeted drug delivery. J. Mater. Chem. B 2015, 3, 651–664. [Google Scholar] [CrossRef]
- Rajender, G.; Giri, P.K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Kumar, G.S.; Roy, R.; Sen, D.; Ghorai, U.K.; Thapa, R.; Mazumder, N.; Saha, S.; Chattopadhyay, K.K. Amino-functionalized graphene quantum dots: Origin of tunable heterogeneous photoluminescence. Nanoscale 2014, 6, 3384. [Google Scholar] [CrossRef]
- Modafferi, V.; Fiore, M.; Fazio, E.; Patanè, S.; Triolo, C.; Santangelo, S.; Ruffo, R.; Neri, F.; Musolino, M.G. Synthesis and characterization of Fe2O3/reduced graphene oxide nanocomposite as a high-performance anode material for sodium-ion batteries. Model. Meas. Control B 2018, 87, 129–134. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giofrè, S.V.; Tiecco, M.; Celesti, C.; Patanè, S.; Triolo, C.; Gulino, A.; Spitaleri, L.; Scalese, S.; Scuderi, M.; Iannazzo, D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials 2020, 10, 2549. https://doi.org/10.3390/nano10122549
Giofrè SV, Tiecco M, Celesti C, Patanè S, Triolo C, Gulino A, Spitaleri L, Scalese S, Scuderi M, Iannazzo D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials. 2020; 10(12):2549. https://doi.org/10.3390/nano10122549
Chicago/Turabian StyleGiofrè, Salvatore V., Matteo Tiecco, Consuelo Celesti, Salvatore Patanè, Claudia Triolo, Antonino Gulino, Luca Spitaleri, Silvia Scalese, Mario Scuderi, and Daniela Iannazzo. 2020. "Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent" Nanomaterials 10, no. 12: 2549. https://doi.org/10.3390/nano10122549
APA StyleGiofrè, S. V., Tiecco, M., Celesti, C., Patanè, S., Triolo, C., Gulino, A., Spitaleri, L., Scalese, S., Scuderi, M., & Iannazzo, D. (2020). Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials, 10(12), 2549. https://doi.org/10.3390/nano10122549