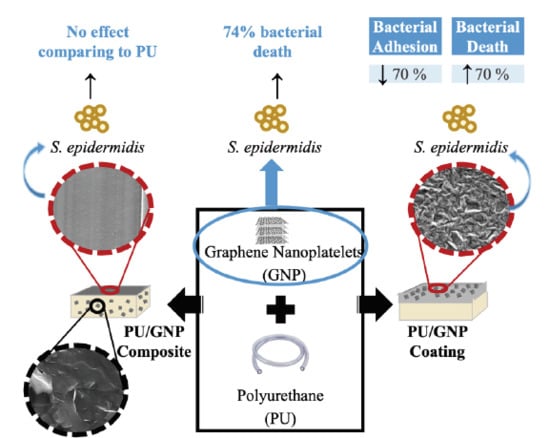
Exposure of Smaller and Oxidized Graphene on Polyurethane Surface Improves its Antimicrobial Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oxidation of Graphene Nanoplatelets (GNP)
2.2. Production of GNP-Containing Polyurethane
2.2.1. Production of PU/GNP Composites
2.2.2. Production of PU/GNP Coatings
2.3. Characterization of GNP Powders and GNP-Containing Polyurethane
2.3.1. Transmission Electron Microscopy (TEM)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Photoelectron spectroscopy (XPS)
2.3.4. X-ray Diffraction (XRD)
2.3.5. Raman Spectroscopy
2.3.6. Water Contact Angle
2.3.7. Rubbing Test
2.4. Antibacterial Activity Assays
2.4.1. Bacterial Strains and Growth Conditions
2.4.2. Antibacterial Activity of GNP Dispersions
2.4.3. Antibacterial Activity of GNP-Containing Polyurethane
3. Results and Discussion
3.1. GNP Powders
3.2. PU/GNP Composites
3.3. PU/GNP Coatings
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mermel, L.A. Prevention of Intravascular Catheter-Related Infections. Ann. Intern. Med. 2000, 132, 391–402. [Google Scholar] [CrossRef]
- Blot, S.I.; Depuydt, P.; Annemans, L.; Benoit, D.; Hoste, E.; De Waele, J.J.; Decruyenaere, J.; Vogelaers, D.; Colardyn, F.; Vandewoude, K.H. Clinical and Economic Outcomes in Critically Ill Patients with Nosocomial Catheter-Related Bloodstream Infections. Clin. Infect. Dis. 2005, 41, 1591–1598. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-Negative Staphylococcal Infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus Epidermidis-the ‘Accidental’ Pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L. Guidelines for the Prevention of Intravascular Catheter-Related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupp, M.E.; Archer, G.L. Coagulase-Negative Staphylococci: Pathogens Associated with Medical Progress. Clin. Infect. Dis. 1994, 19, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.-F.; Dubois, Y.; Minet, C.; Bonadona, A.; Lugosi, M.; Ara-Somohano, C.; Hamidfar-Roy, R.; Schwebel, C. New materials and devices for preventing catheter-related infections. Ann. Intensiv. Care 2011, 1, 34. [Google Scholar] [CrossRef] [Green Version]
- Gloukhoff Wentling, A. Hemodialysis Catheters: Materials, Design and Manufacturing. In Hemodialysis Vascular Access and Peritoneal Dialysis Access; Karger Publishers: Basel, Switzerland, 2004; pp. 112–127. [Google Scholar]
- McKeen, L. Elastomers. In The Effect of Sterilization on Plastics and Elastomers; William Andrew Publishing: Boston, MA, USA, 2012; pp. 319–353. [Google Scholar]
- Schierholz, J.M.; Steinhauser, H.; Rump, A.; Berkels, R.; Pulverer, G. Controlled release of antibiotics from biomedical polyurethanes: Morphological and structural features. Biomaterial 1997, 18, 839–844. [Google Scholar] [CrossRef]
- Falagas, M.E.; Fragoulis, K.; Bliziotis, I.A.; Chatzinikolaou, I. Rifampicin-impregnated central venous catheters: A meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2007, 59, 359–369. [Google Scholar] [CrossRef]
- Tal, M.G.; Ni, N. Selecting Optimal Hemodialysis Catheters: Material, Design, Advanced Features, and Preferences. Tech. Vasc. Interv. Radiol. 2008, 11, 186–191. [Google Scholar] [CrossRef] [PubMed]
- May, R.M.; Magin, C.M.E.; Mann, E.; Drinker, M.C.; Fraser, J.C.A.; Siedlecki, C.; Brennan, A.B.; Reddy, S.T. An engineered micropattern to reduce bacterial colonization, platelet adhesion and fibrin sheath formation for improved biocompatibility of central venous catheters. Clin. Transl. Med. 2015, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Riool, M.; De Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial Peptides in Biomedical Device Manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Muranyi, P.; Schraml, C.; Wunderlich, J. Antimicrobial Efficiency of Titanium Dioxide-Coated Surfaces. J. Appl. Microbiol. 2010, 108, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Zarafu, I.; Turcu, I.; Culiță, D.C.; Petrescu, S.; Popa, M.; Chifiriuc, M.C.; Limban, C.; Telehoiu, A.; Ioniță, P. Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines. Material 2018, 11, 1704. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wen, J.; Gao, Y.; Li, T.; Wang, H.; Yan, H.; Niu, B.; Guo, R. Antibacterial graphene oxide coatings on polymer substrate. Appl. Surf. Sci. 2018, 436, 624–630. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.-T.; Chiong, E.; Tambyah, P.A. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Torreggiani, M.; Scaramuzzi, M.L.; Manini, A.; Castoldi, F.; Serpieri, N.; Maggi, N.; Sileno, G.; Migotto, C.; Esposito, V.; Montagna, F.; et al. Hemodialysis Vascular Access: Everything You Always Wanted to Know About It (but Were Afraid to Ask). J. Nephrol. 2013, 26, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vazquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Swetha, P.D.P.; Manisha, H.; Sudhakaraprasad, K. Graphene and Graphene-Based Materials in Biomedical Science. Part. Part. Syst. Charact. 2018, 35, 1800105. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Comprehensive Application of Graphene: Emphasis on Biomedical Concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; De Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Choudhary, P.; Das, S.K. Bio-Reduced Graphene Oxide as a Nanoscale Antimicrobial Coating for Medical Devices. ACS Omega 2019, 4, 387–397. [Google Scholar] [CrossRef]
- Malmsten, M. Nanomaterials as Antimicrobial Agents. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1053–1075. [Google Scholar]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Carmela Lauriola, M.; Ciasca, G.; Conti, C.; De Spirito, M.; Papi, M. The Graphene Oxide Contradictory Effects against Human Pathogens. Nanotechnology 2017, 28, 152001. [Google Scholar] [CrossRef] [PubMed]
- Notley, S.M.; Crawford, R.J.; Ivanova, E.P. Bacterial Interaction with Graphene Particles and Surfaces. Adv. Graphene Sci. 2013, 99–118. [Google Scholar]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D.F. Graphene Nanocomposite for Biomedical Applications: Fabrication, Antimicrobial and Cytotoxic Investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Han, W.; Wu, Z.; Li, Y.; Wang, Y. Graphene family nanomaterials (GFNs)—promising materials for antimicrobial coating and film: A review. Chem. Eng. J. 2019, 358, 1022–1037. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.; Saltikov, C.; Chen, S. Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef]
- Hegab, H.M.; El Mekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- An, X.L.; Ma, H.B.; Liu, B.; Wang, J.Z. Graphene Oxide Reinforced Polylactic Acid/Polyurethane Antibacterial Composites. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Thakur, S.; Barua, S.; Karak, N. Self-Healable Castor Oil Based Tough Smart Hyperbranched Polyurethane Nanocomposite with Antimicrobial Attributes. RSC Adv. 2015, 5, 2167–2176. [Google Scholar] [CrossRef]
- Tang, X.Z.; Mu, C.Z.; Zhu, W.Y.; Yan, X.L.; Hu, X.; Yang, J.L. Flexible Polyurethane Composites Prepared by Incorporation of Polyethylenimine-Modified Slightly Reduced Graphene Oxide. Carbon 2016, 98, 432–440. [Google Scholar] [CrossRef]
- Sjollema, J.; Zaat, S.A.; Fontaine, V.; Ramstedt, M.; Luginbuehl, R.; Thevissen, K.; Li, J.; Van Der Mei, H.C.; Busscher, H.J. In Vitro Methods for the Evaluation of Antimicrobial Surface Designs. Acta Biomater. 2018, 70, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Goncalves, C.; Sousa, D.M.; Ferreira, A.R.; Moreira, J.A.; Goncalves, I.C.; Magalhaes, F.D. Smaller Particle Size and Higher Oxidation Improves Biocompatibility of Graphene-Based Materials. Carbon 2016, 99, 318–329. [Google Scholar] [CrossRef]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.E.; Pestana, M.; Magalhaes, F.D.; Pinto, A.M.; Goncalves, I.C. Antimicrobial Graphene Nanoplatelets Coatings for Silicone Catheters. Carbon 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, J.A.; Magalhaes, F.D.; Goncalves, I.C. Polymer Surface Adsorption as a Strategy to Improve the Biocompatibility of Graphene Nanoplatelets. Colloids Surf. B Biointerfaces 2016, 146, 818–824. [Google Scholar] [CrossRef]
- Martins, M.C.L.; Wang, D.; Ji, J.; Feng, L.; Barbosa, M.A. Albumin and Fibrinogen Adsorption on Pu–Phema Surfaces. Biomaterials 2003, 24, 2067–2076. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate Chemical Analysis of Oxygenated Graphene-Based Materials Using X-Ray Photoelectron Spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene Oxide and Reduced Graphene Oxide Studied by the Xrd, Tem and Electron Spectroscopy Methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (Mic) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, A.; Colucci, G.; Giorcelli, M.; Frache, A.; Badini, C. Thermal Behavior of Thermoplastic Polymer Nanocomposites Containing Graphene Nanoplatelets. J. Appl. Polym. Sci. 2017, 134, 44814. [Google Scholar] [CrossRef]
- Shams, E.; Yeganeh, H.; Naderi-Manesh, H.; Gharibi, R.; Mohammad Hassan, Z. Polyurethane/Siloxane Membranes Containing Graphene Oxide Nanoplatelets as Antimicrobial Wound Dressings: In Vitro and in Vivo Evaluations. J. Mater. Sci. Mater. Electron. 2017, 28, 75. [Google Scholar] [CrossRef]
- Strankowski, M.; Korzeniewski, P.; Strankowska, J.; Anu, A.S.; Thomas, S. Morphology, Mechanical and Thermal Properties of Thermoplastic Polyurethane Containing Reduced Graphene Oxide and Graphene Nanoplatelets. Material 2018, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Urban, M.; Strankowski, M. Shape Memory Polyurethane Materials Containing Ferromagnetic Iron Oxide and Graphene Nanoplatelets. Material 2017, 10, 1083. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.F.; Lai, S.; Lin, Z.D.; Chen, J.M.; Lin, J. Preparation and Characterization of Gnp/Tpu Composites. Appl. Mech. Mater. 2014, 644, 4760–4762. [Google Scholar] [CrossRef]
- Yadav, S.K.; Cho, J.W. Functionalized Graphene Nanoplatelets for Enhanced Mechanical and Thermal Properties of Polyurethane Nanocomposites. Appl. Surf. Sci. 2013, 266, 360–367. [Google Scholar] [CrossRef]
- Yuan, D.; Pedrazzoli, D.; Pircheraghi, G.; Manas-Zloczower, I. Melt Compounding of Thermoplastic Polyurethanes Incorporating 1d and 2d Carbon Nanofillers. Polym. Plast. Technol. Eng. 2017, 56, 732–743. [Google Scholar] [CrossRef]
- Goncalves, C.; Goncalves, I.C.; Magalhaes, F.D.; Pinto, A.M. Poly(Lactic Acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets Via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Rago, I.; Bregnocchi, A.; Zanni, E.; Aloia, A.G.D.; Angelis, F.D.; Bossu, M.; Bellis, G.D.; Polimeni, A.; Uccelletti, D.; Sarto, M.S. Antimicrobial Activity of Graphene Nanoplatelets against Streptococcus Mutans. Paper presented at the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015. [Google Scholar]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Wang, X.P.; Han, H.Y. A New Function of Graphene Oxide Emerges: Inactivating Phytopathogenic Bacterium Xanthomonas Oryzae Pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial Activity of Dithiothreitol Reduced Graphene Oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Kurantowicz, N.; Sawosz, E.; Jaworski, S.; Kutwin, M.; Strojny, B.; Wierzbicki, M.; Szeliga, J.; Hotowy, A.; Lipinska, L.; Kozinski, R.; et al. Interaction of Graphene Family Materials with Listeria Monocytogenes and Salmonella Enterica. Nanoscale Res. Lett. 2015, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Harper, C.A. Modern Plastics Handbook; McGraw-Hill Professional: New York, NY, USA, 2000. [Google Scholar]
- Pinto, A.M.; Gonçalves, C.; Gonçalves, I.C.; Magalhães, F.D. Effect of biodegradation on thermo-mechanical properties and biocompatibility of poly(lactic acid)/graphene nanoplatelets composites. Eur. Polym. J. 2016, 85, 431–444. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Thampi, S.; Nandkumar, A.M.; Muthuvijayan, V.; Parameswaran, R. Differential Adhesive and Bioactive Properties of the Polymeric Surface Coated with Graphene Oxide Thin Film. ACS Appl. Mater. Interfaces 2017, 9, 4498–4508. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, H.; Zheng, J.; Yu, Q.; Wu, Z.; Yuan, L. Inhibition of protein adsorption and cell adhesion on PNIPAAm-grafted polyurethane surface: Effect of graft molecular weight. Colloids Surf. B Biointerfaces 2011, 85, 26–31. [Google Scholar] [CrossRef]
- Stachelek, S.J.; Alferiev, I.; Choi, H.; Kronsteiner, A.; Uttayarat, P.; Gooch, K.J.; Composto, R.J.; Chen, I.W.; Hebbel, R.P.; Levy, R.J. Cholesterol-Derivatized Polyurethane: Characterization and Endothelial Cell Adhesion. J. Biomed. Mater. Res. A 2005, 72, 200–212. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Li, Y.; Zhang, J.; Ding, Y. The surface properties and corrosion resistance of fluorinated polyurethane coatings. J. Fluor. Chem. 2015, 176, 14–19. [Google Scholar] [CrossRef]
- Pinto, A.M.; Martins, J.M.; Moreira, J.A.; Mendes, A.M.; Magalhães, F.D. Dispersion of graphene nanoplatelets in poly(vinyl acetate) latex and effect on adhesive bond strength. Polym. Int. 2013, 62, 928–935. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, S.; Gonçalves, I.C.; Gama, F.M.; Mendes, A.M.; Magalhães, F.D.; Gama, M. Biocompatibility of poly(lactic acid) with incorporated graphene-based materials. Colloids Surf. B Biointerfaces 2013, 104, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Pant, H.R.; Pokharel, P.; Joshi, M.K.; Adhikari, S.; Kim, H.J.; Park, C.H.; Kim, C.S.; Pokherel, P. Processing and characterization of electrospun graphene oxide/polyurethane composite nanofibers for stent coating. Chem. Eng. J. 2015, 270, 336–342. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Re, G.L.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Lu, X.; Kaneda, M.; Zhang, W.; Bernstein, R.; Ma, J.; Elimelech, M. Graphene Oxide-Functionalized Membranes: The Importance of Nanosheet Surface Exposure for Biofouling Resistance. Environ. Sci. Technol. 2019, 54, 517–526. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef] [Green Version]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; Rodrigues, D.; Advincula, R.C.; De Leon, A.C.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing Bacterial Interaction with Copper Surfaces through Graphene and Hexagonal-Boron Nitride Coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, I.; Henriques, P.C.; Gomes, R.N.; Pinto, A.M.; Pestana, M.; Magalhães, F.D.; Gonçalves, I.C. Exposure of Smaller and Oxidized Graphene on Polyurethane Surface Improves its Antimicrobial Performance. Nanomaterials 2020, 10, 349. https://doi.org/10.3390/nano10020349
Borges I, Henriques PC, Gomes RN, Pinto AM, Pestana M, Magalhães FD, Gonçalves IC. Exposure of Smaller and Oxidized Graphene on Polyurethane Surface Improves its Antimicrobial Performance. Nanomaterials. 2020; 10(2):349. https://doi.org/10.3390/nano10020349
Chicago/Turabian StyleBorges, Inês, Patrícia C. Henriques, Rita N. Gomes, Artur M. Pinto, Manuel Pestana, Fernão D. Magalhães, and Inês C. Gonçalves. 2020. "Exposure of Smaller and Oxidized Graphene on Polyurethane Surface Improves its Antimicrobial Performance" Nanomaterials 10, no. 2: 349. https://doi.org/10.3390/nano10020349
APA StyleBorges, I., Henriques, P. C., Gomes, R. N., Pinto, A. M., Pestana, M., Magalhães, F. D., & Gonçalves, I. C. (2020). Exposure of Smaller and Oxidized Graphene on Polyurethane Surface Improves its Antimicrobial Performance. Nanomaterials, 10(2), 349. https://doi.org/10.3390/nano10020349