Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Panel of MWCNT and Evaluation of Physical Properties
2.2. Evaluation of Chemical Properties
2.2.1. Purity and Metal Impurities of MWCNT Powders
2.2.2. Soluble Metal Impurities of MWCNTs
2.3. Evaluation of Reactive Oxygen Species (ROS) Generation Potentials of MWCNTs or MWCNT-Free Supernatants
2.4. Preparation of MWCNTs or MWCNT-Free Soluble Fractions for In Vivo Experiments
2.5. Intratracheal Instillation of MWCNTs or MWCNT-Free Soluble Fractions
2.6. Preparation of Bronchoalveolar Lavage Fluid (BALF)
2.7. Measurement of Lactate Dehydrogenase (LDH) and Total Protein in BALF
2.8. Statistical Analysis
3. Results
3.1. Physical Characteristics of MWCNTs
3.2. Chemical Characteristics of MWCNTs
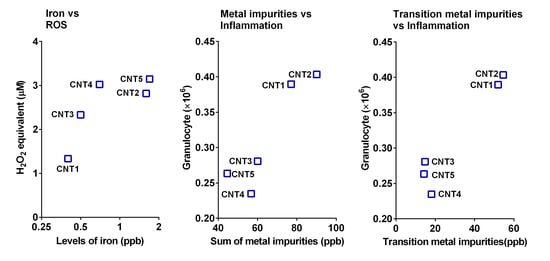
3.3. ROS Generation Potential of MWCNTs or Soluble Fractions
3.4. Acute Lung Inflammation by MWCNTs
3.5. Effect of Soluble Metal Impurities on the Acute Inflammogenic Potential of MWCNTs
3.6. The Parameter Triggering the Acute Lung Inflammation of Soluble Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Donaldson, K.; Schinwald, A.; Murphy, F.; Cho, W.S.; Duffin, R.; Tran, L.; Poland, C. The biologically effective dose in inhalation nanotoxicology. Acc. Chem. Res. 2013, 46, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Okazaki, Y.; Chew, S.H.; Misawa, N.; Yamashita, Y.; Akatsuka, S.; Ishihara, T.; Yamashita, K.; Yoshikawa, Y.; Yasui, H.; et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, E1330–E1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamon, L.; Asturiol, D.; Richarz, A.; Joossens, E.; Graepel, R.; Aschberger, K.; Worth, A. Grouping of nanomaterials to read-across hazard endpoints: from data collection to assessment of the grouping hypothesis by application of chemoinformatic techniques. Part. Fibre Toxicol. 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Lamon, L.; Aschberger, K.; Asturiol, D.; Richarz, A.; Worth, A. Grouping of nanomaterials to read-across hazard endpoints: a review. Nanotoxicology 2019, 13, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.; Rasmussen, K.; Jantunen, P.; Aicher, L.; Alessandrelli, M.; Bernauer, U.; Bleeker, E.A.J.; Bouillard, J.; Di Prospero Fanghella, P.; Draisci, R.; et al. Insights into possibilities for grouping and read-across for nanomaterials in EU chemicals legislation. Nanotoxicology 2019, 13, 119–141. [Google Scholar] [CrossRef] [Green Version]
- Duke, K.S.; Taylor-Just, A.J.; Ihrie, M.D.; Shipkowski, K.A.; Thompson, E.A.; Dandley, E.C.; Parsons, G.N.; Bonner, J.C. STAT1-dependent and-independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Part. Fibre Toxicol. 2017, 14, 26. [Google Scholar] [CrossRef]
- Lee, D.K.; Jeon, S.; Han, Y.; Kim, S.H.; Lee, S.; Yu, I.J.; Song, K.S.; Kang, A.; Yun, W.S.; Kang, S.M.; et al. Threshold Rigidity Values for the Asbestos-like Pathogenicity of High-Aspect-Ratio Carbon Nanotubes in a Mouse Pleural Inflammation Model. ACS Nano 2018, 12, 10867–10879. [Google Scholar] [CrossRef]
- Schinwald, A.; Murphy, F.A.; Prina-Mello, A.; Poland, C.A.; Byrne, F.; Movia, D.; Glass, J.R.; Dickerson, J.C.; Schultz, D.A.; Jeffree, C.E.; et al. The threshold length for fiber-induced acute pleural inflammation: shedding light on the early events in asbestos-induced mesothelioma. Toxicol. Sci. 2012, 128, 461–470. [Google Scholar] [CrossRef] [Green Version]
- IARC. Some nanomaterials and some fibres. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Internatioanl Agency for Research on Cancer: Lyon, France, 2017; Volume 111, pp. 35–214. [Google Scholar]
- Sager, T.M.; Wolfarth, M.W.; Andrew, M.; Hubbs, A.; Friend, S.; Chen, T.H.; Porter, D.W.; Wu, N.; Yang, F.; Hamilton, R.F.; et al. Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57BL/6 mouse model. Nanotoxicology 2014, 8, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Galano, A. Carbon nanotubes: Promising agents against free radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Pinero, E.; Beguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Delos, M.; Panin, N.; Rabolli, V.; Huaux, F.; Lison, D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol. Sci. 2009, 110, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Miyahara, Y. What amount of metallic impurities in carbon nanotubes is small enough not to dominate their redox properties? Nanoscale 2009, 1, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, R.F., Jr.; Buford, M.; Xiang, C.; Wu, N.; Holian, A. NLRP3 inflammasome activation in murine alveolar macrophages and related lung pathology is associated with MWCNT nickel contamination. Inhal. Toxicol. 2012, 24, 995–1008. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Li, W.; Li, Y.; Li, B.; Du, J.; Qiu, Y.; Liu, Y.; Gao, Y.; Chai, Z.; Chen, C. Significance and systematic analysis of metallic impurities of carbon nanotubes produced by different manufacturers. J. Nanosci. Nanotechnol. 2011, 11, 2389–2397. [Google Scholar] [CrossRef]
- Lee, N.; Lim, C.H.; Kim, T.; Son, E.K.; Chung, G.S.; Rho, C.J.; Lee, S.R.; Yu, I.J. Which Hazard Category Should Specific Nanomaterials or Groups of Nanomaterials be Assigned to and How; World Health Organization: Geneva, Switzerland, 2017; Available online: https://pdfs.semanticscholar.org/ece6/da58902ed3a7d91f58da90abe5dd8b196dfc.pdf (accessed on 20 February 2020).
- Morimoto, Y.; Horie, M.; Kobayashi, N.; Shinohara, N.; Shimada, M. Inhalation toxicity assessment of carbon-based nanoparticles. Acc. Chem. Res. 2013, 46, 770–781. [Google Scholar] [CrossRef]
- International Organization for Standardization. TECHNICAL SPECIFICATION ISO/TS Nanotechnologies-Characterization of Multiwalled Carbon Nanotubes-Mesoscopic Shape Factors; International Organization for Standardization: Geneva, Switzerland, 2017; Available online: https://www.iso.org/standard/69549.html (accessed on 20 February 2020).
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that influence the specific surface areas of carbon nanotubes and nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar]
- Rahman, L.; Jacobsen, N.R.; Aziz, S.A.; Wu, D.; Williams, A.; Yauk, C.L.; White, P.; Wallin, H.; Vogel, U.; Halappanavar, S. Multi-walled carbon nanotube-induced genotoxic, inflammatory and pro-fibrotic responses in mice: Investigating the mechanisms of pulmonary carcinogenesis. Mutat. Res. 2017, 823, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Stopford, W.; Turner, J.; Cappellini, D.; Brock, T. Bioaccessibility testing of cobalt compounds. J. Environ. Monit. 2003, 5, 675–680. [Google Scholar] [CrossRef]
- Han, Y.; Lee, D.K.; Kim, S.H.; Lee, S.; Jeon, S.; Cho, W.S. High inflammogenic potential of rare earth oxide nanoparticles: The New Hazardous Entity. Nanotoxicology 2018, 12, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, S.H.; Jeong, J.; Han, Y.; Kim, S.H.; Lee, D.K.; Lee, H.S.; Chung, S.T.; Jeong, J.; Roh, C.; et al. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part. Fibre Toxicol. 2016, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles: The role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultquist, G.; Seo, M.; Leitner, T.; Leygraf, C.; Sato, N. The dissolution behaviour of iron, chromium, molybdenum and copper from pure metals and from ferritic stainless steels. Corrosion. Science 1987, 27, 937–946. [Google Scholar] [CrossRef]
- Tamoto, S.; Tabelin, C.B.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Short and long term release mechanisms of arsenic, selenium and boron from a tunnel-excavated sedimentary rock under in situ conditions. J. Contam. Hydrol. 2015, 175, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.L.; Rasmussen, P.E.; Chenier, M. Determination of Metal Impurities in Carbon Nanotubes Sampled Using Surface Wipes. J. Anal. Methods Chem. 2016, 2016, 3834292. [Google Scholar] [CrossRef]
- Ge, C.; Li, Y.; Yin, J.-J.; Liu, Y.; Wang, L.; Zhao, Y.; Chen, C. The contributions of metal impurities and tube structure to the toxicity of carbon nanotube materials. NPG Asia Mater. 2012, 4, e32. [Google Scholar] [CrossRef] [Green Version]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Lu, H.; Zhou, M.; Chai, Z.; Hu, Y. Iron-induced generation of mitochondrial ROS depends on AMPK activity. Biometals 2017, 30, 623–628. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 1995, 82, 969–974. [Google Scholar] [CrossRef]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free. Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Rushton, E.K.; Jiang, J.; Leonard, S.S.; Eberly, S.; Castranova, V.; Biswas, P.; Elder, A.; Han, X.; Gelein, R.; Finkelstein, J.; et al. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J. Toxicol. Environ. Health Part A 2010, 73, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Aldieri, E.; Fenoglio, I.; Cesano, F.; Gazzano, E.; Gulino, G.; Scarano, D.; Attanasio, A.; Mazzucco, G.; Ghigo, D.; Fubini, B. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J. Toxicol. Environ. Health Part A 2013, 76, 1056–1071. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Andreozzi, G.B.; Tomatis, M.; Turci, F. Iron from a geochemical viewpoint. Understanding toxicity/pathogenicity mechanisms in iron-bearing minerals with a special attention to mineral fibers. Free Radic. Biol. Med. 2019, 133, 21–37. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of transition metal oxide nanoparticles: Recent insights from in vitro studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.M.; Doudrick, K.; Franzi, L.M.; TeeSy, C.; Anderson, D.S.; Wu, Z.; Mitra, S.; Vu, V.; Dutrow, G.; Evans, J.E.; et al. Instillation versus inhalation of multiwalled carbon nanotubes: exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano 2014, 8, 8911–8931. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Hamilton, R.F.; Bonner, J.C.; Crandall, E.D.; Elder, A.; Fazlollahi, F.; Girtsman, T.A.; Kim, K.; Mitra, S.; Ntim, S.A.; et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect. 2013, 121, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Vitkina, T.I.; Yankova, V.I.; Gvozdenko, T.A.; Kuznetsov, V.L.; Krasnikov, D.V.; Nazarenko, A.V.; Chaika, V.V.; Smagin, S.V.; Tsatsakis, A.Μ.; Engin, A.B.; et al. The impact of multi-walled carbon nanotubes with different amount of metallic impurities on immunometabolic parameters in healthy volunteers. Food Chem. Toxicol. 2016, 87, 138–147. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Xiang, C.; Li, M.; Ka, I.; Yang, F.; Ma, D.; Porter, D.W.; Wu, N.; Holian, A. Purification and sidewall functionalization of multiwalled carbon nanotubes and resulting bioactivity in two macrophage models. Inhal Toxicol. 2013, 25, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| MWCNTs | Diameter (nm) | Length (µm) | Rigidity | BET (m2/g) | Raman (IG/ID) | |
|---|---|---|---|---|---|---|
| Db | SBPL | |||||
| CNT1 | 16.37 ± 0.2 | 10–50 a | 0.49 ± 0.1 | 0.54 ± 0.0 | 218.26 | 0.85 |
| CNT2 | 15.64 ± 0.1 | 1–25 a | 0.42 ± 0.0 | 0.48 ± 0.0 | 194.03 | 1.05 |
| CNT3 | 7.75 ± 0.1 | 7.55 a | 0.47 ± 0.0 | 0.49 ± 0.0 | 675.44 | 0.64 |
| CNT4 | 16.7 ± 0.2 | 3.55 a | 0.66 ± 0.0 | 0.86 ± 0.0 | 224.90 | 0.92 |
| CNT5 | 58.3 ± 1.0 | 10.02 ± 0.3 | 0.99 ± 0.0 | 1.19 ± 0.0 | 28.2 | 1.01 |
| CNT1 | CNT2 | CNT3 | CNT4 | CNT5 | |
|---|---|---|---|---|---|
| Purity (%) by TGA | >90 | >95 | >99 | 94.9 | >99 a |
| Purity (%) by ICP-MS | >95 | >99 | >99 | >95 | >99 b |
| Major metal impurities (% by weight) | Fe: 0.84 | Al: 0.12 | Al: 0.15 | Al < 4 | Mg < 0.0002 b |
| Al: 0.74 | Fe: 0.08 | Mg: 0.14 | Fe < 2 | Al < 0.009 b | |
| Co: 0.28 | Cu: 0.01 | Co: 0.05 | Co < 2 | Fe < 0.04 b | |
| Ni: 0.0064 | Cu: 0.01 | Ni < 0.0001 b |
| Metals | Property | CNT1 | CNT2 | CNT3 | CNT4 | CNT5 |
|---|---|---|---|---|---|---|
| Al | Other metals | 2.3 | 9.1 | 14.7 | 14.7 | 4.4 |
| As | Metalloids | 5.8 | 7.9 | 7.7 | 7.5 | 3.3 |
| B | Metalloids | 14.5 | 15.6 | 18 | 13.9 | 19.3 |
| Ba | Alkaline metals | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 |
| Co | Transition metals | 5.5 | 6.1 | 2.8 | 5.3 | 0.0 |
| Cr | Transition metals | 0 | 0.1 | 0 | 0 | 0.1 |
| Cu | Transition metals | 0.1 | 0.4 | 0.2 | 0.1 | 0.2 |
| Fe | Transition metals | 0.4 | 1.6 | 0.5 | 0.7 | 1.7 |
| Ga | other metals | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Li | Alkali metals | 0 | 0.2 | 0.2 | 0.1 | 0.3 |
| Mn | Transition metals | 0.1 | 0.2 | 0 | 0 | 0 |
| Mo | Transition metals | 35.9 | 35.9 | 0.2 | 0.2 | 0.1 |
| Ni | Transition metals | 0.2 | 0.2 | 0.4 | 0.1 | 0.2 |
| Rb | Alkali metals | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 |
| Sb | Metalloids | 0.6 | 0.5 | 1.7 | 0.5 | 0.5 |
| Se | Nonmetals | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 |
| Sn | other metals | 0.1 | 0.1 | 0 | 0.1 | 0 |
| Sr | Alkaline metals | 1.1 | 1.1 | 1.8 | 1.1 | 1.4 |
| Ti | Transition metals | 0.7 | 0.6 | 0.9 | 0.7 | 0.8 |
| W | Transition metals | 0.6 | 0.3 | 0.2 | 0.2 | 0.1 |
| Zn | Transition metals | 8.5 | 9.2 | 9.6 | 10.8 | 11.1 |
| Nanomaterials | Experimental Model | Toxicity | Reference |
|---|---|---|---|
| O-MWCNT (4.5% Ni, 0.8% Fe) P-MWCNT (1.8% Ni, 0.1% Fe) F-MWCNT (negligible Ni and Fe) | Instillation and inhalation to male SD rats | Inflammation was produced in the order of O-, P-, and F-MWCNT | [41] |
| Nine different types of MWCNT | Instillation | The magnitude of toxicity was strongly associated with the Ni contamination on the particle | [16] |
| O-MWCNT (4.5% Ni, 0.8% Fe) P-MWCNT (1.8% Ni, 0.1% Fe) F-MWCNT (negligible Ni and Fe) | In vitro: BEAS-2B, RLE-6TN, and THP-1 | The levels of IL-1β in THP-1 cells were in the order of O-, P-, and F-MWCNT | [42] |
| Purified MWCNT (Co 0.07%, Fe 0.16%, Mg 0.05%) Unpurified MWCNT (Co 1.3%, Fe 2.4%, Mg 2.5%) | In vitro: Venous blood of healthy human volunteers, A549, and HaCaT | Unpurified MWCNT showed higher toxicity than purified MWCNT by increasing the oxidation reactions | [43] |
| Raw MWCNT (Fe 0.08%, Ni 2.2%) Purified MWCNT (Ni 0.96%) | In vitro: alveolar macrophages from C57BL/6 mouse, THP-1 | Purified MWCNT showed less toxicity and inflammasome activation than raw MWCNT | [44] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-K.; Jeon, S.; Jeong, J.; Yu, I.J.; Song, K.S.; Kang, A.; Yun, W.S.; Kim, J.S.; Cho, W.-S. Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes. Nanomaterials 2020, 10, 379. https://doi.org/10.3390/nano10020379
Lee D-K, Jeon S, Jeong J, Yu IJ, Song KS, Kang A, Yun WS, Kim JS, Cho W-S. Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes. Nanomaterials. 2020; 10(2):379. https://doi.org/10.3390/nano10020379
Chicago/Turabian StyleLee, Dong-Keun, Soyeon Jeon, Jiyoung Jeong, Il Je Yu, Kyung Seuk Song, Aeyeon Kang, Wan Soo Yun, Jong Sung Kim, and Wan-Seob Cho. 2020. "Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes" Nanomaterials 10, no. 2: 379. https://doi.org/10.3390/nano10020379
APA StyleLee, D. -K., Jeon, S., Jeong, J., Yu, I. J., Song, K. S., Kang, A., Yun, W. S., Kim, J. S., & Cho, W. -S. (2020). Potential Role of Soluble Metal Impurities in the Acute Lung Inflammogenicity of Multi-Walled Carbon Nanotubes. Nanomaterials, 10(2), 379. https://doi.org/10.3390/nano10020379