Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Gas Sensor Fabrication
2.3. Structural Characterization
2.4. Performance Measurements
3. Results and discussion
3.1. Morphology and Composition of Materials
3.2. Performance for Acetone Analysis
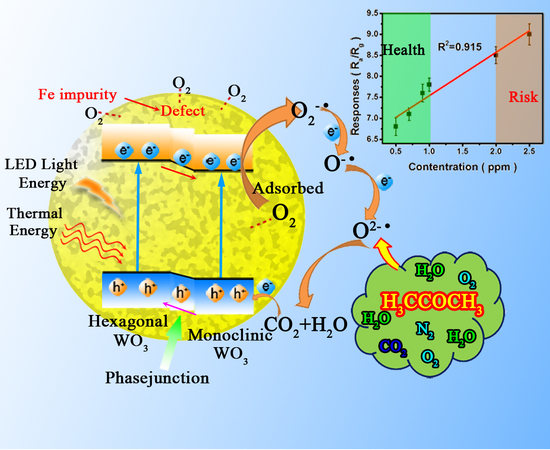
3.3. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Staerz, A.; Weimar, U.; Barsan, N. Understanding the potential of WO3 based sensors for breath analysis. Sensors 2016, 16, 1815. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A. Sensors for enhanced detection of acetone as a potential tool for noninvasive diabetes monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Lan, C.; Wei, R.; Ho, J.C. Transparent metal-oxide nanowires and their applications in harsh electronics. J. Mater. Chem. C 2019, 7, 202–217. [Google Scholar] [CrossRef]
- Wang, X.-F.; Ma, W.; Jiang, F.; Cao, E.-S.; Sun, K.-M.; Cheng, L.; Song, X.-Z. Prussian blue analogue derived porous NiFe2O4 nanocubes for low-concentration acetone sensing at low working temperature. Chem. Eng. J. 2018, 338, 504–512. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Zhang, L.; Ren, J.; Wang, J.-C.; Yao, H.-C.; Li, Z.-J. Highly enhanced acetone sensing performance of porous C-doped WO3 hollow spheres by carbon spheres as templates. Sens. Actuators B 2017, 239, 597–607. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, X.-Y.; Zhao, Z.-H.; Li, L.; Zhang, L.; Yao, H.-C.; Wang, J.-S.; Li, Z.-J. Highly sensitive and selective acetone sensor based on C-doped WO3 for potential diagnosis of diabetes mellitus. Sens. Actuators B 2014, 199, 210–219. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Zhang, P.; Hu, J. High sensing properties of 3 wt% Pd doped SmFe1−xMgxO3 nanocrystalline powders to acetone vapor with ultralow concentrations under light illumination. ACS Appl. Mater. Interfaces 2018, 10, 15558–15564. [Google Scholar] [CrossRef]
- Rydosz, A. A negative correlation between blood glucose and acetone measured in healthy and type-1 diabetes mellitus patient breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef]
- Amann, A.; Smith, D. Volatile Biomarkers. In Non-Invasive Diagnosis in Physiology and Medicine; Elsevier: New York, NY, USA, 2013. [Google Scholar]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.-S.; Matras-Postolek, K.; Yang, P. ZnO nanoflowers with single crystal structure towards enhanced gas sensing and photocatalysis. Phys. Chem. Chem. Phys. 2015, 17, 30300–30306. [Google Scholar] [CrossRef]
- Lim, C.-J.; Park, J.-W. Luminescent oxygen-sensing films with improved sensitivity based on light scattering by TiO2 particles. Sens. Actuators B 2017, 253, 934–941. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Photoactivated mixed in-plane and edge-enriched p-type MoS2 flake-based NO2 sensor working at room temperature. ACS Sens. 2018, 3, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Xiao, S.; Wang, X.; Sun, L.; Li, H.; Xie, W.; Li, Q.; Zhang, Q.; Wang, T. Low-temperature H2S detection with hierarchical Cr-doped WO3 microspheres. ACS Appl. Mater. Interfaces 2016, 8, 9674–9683. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhu, Z.; Guo, T.; Li, H.; Xue, Q. Synthesis of nanowire bundle-like WO3-W18O49 heterostructures for highly sensitive NH3 sensor application. J. Hazard. Mater. 2018, 353, 290–299. [Google Scholar] [CrossRef]
- Lee, E.; Yoon, Y.S.; Kim, D.J. Two-dimensional transition metal dichalcogenides and metal oxide hybrids for gas sensing. ACS Sens. 2018, 3, 2045–2060. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe-Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors-A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Xie, W.; Li, H.; Zhou, R.; Li, Q.; Wang, T. Enhanced selective acetone sensing characteristics based on Co-doped WO3 hierarchical flower-like nanostructures assembled with nanoplates. Sens. Actuators B 2016, 235, 614–621. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.; Guan, N.; Li, L.; Gong, J. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 2015, 27, 1580–1586. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.-J. Preparation of 2D WO3 nanomaterials with enhanced catalytic activities: Current status and perspective. ChemBioEng Rev. 2015, 2, 335–350. [Google Scholar] [CrossRef]
- Balázsi, C.; Wang, L.; Zayim, E.O.; Szilágyi, I.M.; Sedlacková, K.; Pfeifer, J.; Tóth, A.L.; Gouma, P.-I. Nanosize hexagonal tungsten oxide for gas sensing applications. J. Eur. Ceram. Soc. 2008, 28, 913–917. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, X.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an alpha-beta phase junction on Ga2O3. Angew. Chem. Int. Ed. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Sukunta, J.; Wisitsoraat, A.; Tuantranont, A.; Jaruwongrungsee, K.; Phanichphant, S.; Liewhiran, C. Mechanistic roles of substitutional Fe dopants on catalytic acetylene-sensing process of flame-made SnO2 nanoparticles. Arabian J. Chem. 2020, 13, 3043–3059. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, B.; Zhou, Q.; He, Y.; Wang, Z.; Radacsi, N. Fe-doped ZnO/reduced graphene oxide nanocomposite with synergic enhanced gas sensing performance for the effective detection of formaldehyde. ACS Omega 2019, 4, 10252–10262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, T.G.; Bari, N.E.; Ionescu, R.; Smulko, J.; Bouchikhi, B. Exhaled breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuators B 2018, 273, 1719–1729. [Google Scholar] [CrossRef]
- Chen, X.; Deng, N.; Zhang, X.; Yang, Y.; Li, J.; Hong, B.; Fang, J.; Xu, J.; Jin, D.; Peng, X.; et al. Preparation of Fe-doped In2O3 gas sensing semiconductor by one-step impregnation with enhanced ethanol sensing. Chem. Phys. Lett. 2019, 722, 93–103. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Wang, M.-D.; Wang, Y.-F.; Hu, J.-Y.; Zhu, Y.; Zhang, Y.X.; Li, Z.-J.; Yao, H.-C. Iron and carbon codoped WO3 with hierarchical walnut-like microstructure for highly sensitive and selective acetone sensor. Sens. Actuators B 2018, 256, 27–37. [Google Scholar] [CrossRef]
- Staerz, A.; Liu, Y.; Geyik, U.; Brinkmann, H.; Weimar, U.; Zhang, T.; Barsan, N. The effect of platinum loading on WO3 based sensors. Sens. Actuators B 2019, 291, 378–384. [Google Scholar] [CrossRef]
- Zeb, S.; Peng, X.; Yuan, G.; Zhao, X.; Qin, C.; Sun, G.; Nie, Y.; Cui, Y.; Jiang, X. Controllable synthesis of ultrathin WO3 nanotubes and nanowires with excellent gas sensing performance. Sens. Actuators B 2020, 305, 127435. [Google Scholar] [CrossRef]
- Qi, J.; Chen, K.; Xing, Y.; Fan, H.; Zhao, H.; Yang, J.; Li, L.; Yan, B.; Zhou, J.; Guo, L.; et al. Application of 3D hierarchical monoclinic-type structural Sb-doped WO3 towards NO2 gas detection at low temperature. Nanoscale 2018, 10, 7440–7450. [Google Scholar] [CrossRef]
- Shi, W.; Guo, X.; Cui, C.; Jiang, K.; Li, Z.; Qu, L.; Wang, J.-C. Controllable synthesis of Cu2O decorated WO3 nanosheets with dominant (0 0 1) facets for photocatalytic CO2 reduction under visible-light irradiation. Appl. Catal. B 2019, 243, 236–242. [Google Scholar] [CrossRef]
- Shen, Y.; Bi, H.; Li, T.; Zhong, X.; Chen, X.; Fan, A.; Wei, D. Low-temperature and highly enhanced NO2 sensing performance of Au-functionalized WO3 microspheres with a hierarchical nanostructure. Appl. Surf. Sci. 2018, 434, 922–931. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wang, F.; Chen, X.; Feng, Z.; Li, C. K2SO4-assisted hexagonal/monoclinic WO3 phase junction for efficient photocatalytic degradation of RhB. ACS Appl. Energy Mater. 2018, 1, 2067–2077. [Google Scholar] [CrossRef]
- Xie, D.; Li, H.-H.; Shi, Y.-H.; Diao, W.-Y.; Jiang, R.; Sun, H.-Z.; Wu, X.-L.; Li, W.; Fan, C.-Y.; Zhang, J.-P. Micro/nanoengineered a-Fe2O3 nanoaggregate conformably enclosed by ultrathin N-doped carbon shell for ultrastable lithium storage and insight into phase evolution mechanism. Chem. Eur. J. 2020, 26, 853–862. [Google Scholar] [CrossRef]
- Li, S.; Dong, G.; Hailili, R.; Yang, L.; Li, Y.; Wang, F.; Zeng, Y.; Wang, C. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B 2016, 190, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Hu, X.; Ji, H.; Chang, F.; Luo, Y. Simultaneous photocatalytic Cr(VI) reduction and 2,4,6-TCP oxidation over g-C3N4 under visible light irradiation. Catal. Today 2014, 224, 34–40. [Google Scholar] [CrossRef]
- Behera, A.; Mansingh, S.; Das, K.K.; Parida, K. Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr(VI) reduction. J. Colloid Interface Sci. 2019, 544, 96–111. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials 2020, 10, 398. https://doi.org/10.3390/nano10020398
Wang J-C, Shi W, Sun X-Q, Wu F-Y, Li Y, Hou Y. Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials. 2020; 10(2):398. https://doi.org/10.3390/nano10020398
Chicago/Turabian StyleWang, Ji-Chao, Weina Shi, Xue-Qin Sun, Fang-Yan Wu, Yu Li, and Yuxia Hou. 2020. "Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction" Nanomaterials 10, no. 2: 398. https://doi.org/10.3390/nano10020398
APA StyleWang, J. -C., Shi, W., Sun, X. -Q., Wu, F. -Y., Li, Y., & Hou, Y. (2020). Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials, 10(2), 398. https://doi.org/10.3390/nano10020398