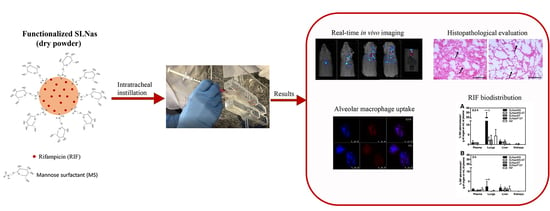
In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SLNas Preparation
2.3. Morphology, Size, and Z-Potential
2.4. HPLC Analysis
2.5. Drug Loading Levels
2.6. In Vitro Release
2.7. In Vivo Study
2.7.1. Animals
2.7.2. Real-Time Fluorescence Imaging
2.7.3. Lung Section Analysis
2.7.4. Rifampicin Biodistribution
2.7.5. Actual Inhaled Drug Dose
2.7.6. Bronchoalveolar Lavage Fluid
2.7.7. Plasma and Organs
2.8. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Properties
3.2. In Vivo Study
3.2.1. Fluorescence Imaging on Mice Whole-Body and Excised Organs
3.2.2. SLNas Biodistribution in the Pulmonary Region
3.2.3. SLNas Biodistribution in Extra-Pulmonary Regions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Johnson, J.D.; Hand, W.L.; Francis, J.B.; King-Thompson, N.; Corwin, R.W. Antibiotic uptake by alveolar macrophages. J. Lab. Clin. Med. 1980, 95, 429–439. [Google Scholar] [PubMed]
- Hirota, K.; Hasegawa, T.; Nakajima, T.; Inagawa, H.; Kohchi, C.; Soma, G.-I.; Makino, K.; Terada, H. Delivery of rifampicin–PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J. Control. Release 2010, 142, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J.; Mansour, H.M. Inhalation Aerosols|Physical and Biological Basis for Therapy, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Maretti, E.; Costantino, L.; Buttini, F.; Rustichelli, C.; Leo, E.; Truzzi, E.; Iannuccelli, V. Newly synthesized surfactants for surface mannosylation of respirable SLN assemblies to target macrophages in tuberculosis therapy. Drug Deliv. Transl. Res. 2019, 9, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, N.P.; Solanki, H. Pulmonary drug delivery system: Review. Int. J. Appl. Pharm. 2013, 5, 7–10. [Google Scholar]
- Pinheiro, M.; Ribeiro, R.; Vieira, A.; Andrade, F.; Reis, S. Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. Drug Des Devel Ther 2016, 10, 2467–2475. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Bihari, S.; Prakash, S. Low-dose inhaled versus standard dose oral form of anti-tubercular drugs: Concentrations in bronchial epithelial lining fluid, alveolar macrophage and serum. J. Postgrad. Med. 2008, 54, 245–246. [Google Scholar]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef]
- Sharma, R.; Muttil, P.; Yadav, A.B.; Rath, S.K.; Bajpai, V.K.; Mani, U.; Misra, A. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 2007, 59, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Iannuccelli, V.; Maretti, E. Inhaled Micro- or Nanoparticles: Which are the Best for Intramacrophagic Antiinfectious Therapies? J. Infect. Dis. Diagn 2015, 1, e102. [Google Scholar] [CrossRef]
- Takenaga, M.; Ohta, Y.; Tokura, Y.; Hamaguchi, A.; Igarashi, R.; Disratthakit, A.; Doi, N. Lipid Microsphere Formulation Containing Rifampicin Targets Alveolar Macrophages. Drug Delivery 2008, 15, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Rossi, T.; Bondi, M.; Croce, M.A.; Hanuskova, M.; Leo, E.; Sacchetti, F.; Iannuccelli, V. Inhaled Solid Lipid Microparticles to target alveolar macrophages for tuberculosis. Int. J. Pharm. 2014, 462, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Rustichelli, C.; Lassinantti Gualtieri, M.; Costantino, L.; Siligardi, C.; Miselli, P.; Buttini, F.; Montecchi, M.; Leo, E.; Truzzi, E.; et al. The Impact of Lipid Corona on Rifampicin Intramacrophagic Transport Using Inhaled Solid Lipid Nanoparticles Surface-Decorated with a Mannosylated Surfactant. Pharmaceutics 2019, 11, 508. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Kaur, J.; Kumar, K.; Yadav, A.B.; Misra, A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob. Agents Chemother. 2008, 52, 3195–3201. [Google Scholar] [CrossRef] [Green Version]
- Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Thorley, A.J.; Ruenraroengsak, P.; Potter, T.E.; Tetley, T.D. Critical determinants of uptake and translocation of nanoparticles by the human pulmonary alveolar epithelium. ACS Nano 2014, 8, 11778–11789. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef] [Green Version]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano- and microparticles for drug delivery. Glob Cardiol Sci. Pract. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Maretti, E.; Rustichelli, C.; Romagnoli, M.; Balducci, A.G.; Buttini, F.; Sacchetti, F.; Leo, E.; Iannuccelli, V. Solid Lipid Nanoparticle assemblies (SLNas) for an anti-TB inhalation treatment—A Design of Experiments approach to investigate the influence of pre-freezing conditions on the powder respirability. Int. J. Pharm. 2016, 511, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhou, M.; Tu, J.; Sun, C. Design and validation of a simple device for insufflation of dry powders in a mice model. Eur. J. Pharm. Sci. 2018, 123, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Aragao-Santiago, L.; Hillaireau, H.; Grabowski, N.; Mura, S.; Nascimento, T.L.; Dufort, S.; Coll, J.-L.; Tsapis, N.; Fattal, E. Compared in vivo toxicity in mice of lung delivered biodegradable and non-biodegradable nanoparticles. Nanotoxicology 2016, 10, 292–302. [Google Scholar] [CrossRef]
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1. [Google Scholar]
- Ganguly, K.; Ettehadieh, D.; Upadhyay, S.; Takenaka, S.; Adler, T.; Karg, E.; Krombach, F.; Kreyling, W.G.; Schulz, H.; Schmid, O.; et al. Early pulmonary response is critical for extra-pulmonary carbon nanoparticle mediated effects: Comparison of inhalation versus intra-arterial infusion exposures in mice. Part. Fibre. Toxicol. 2017, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-B.; Watts, A.B.; Peters, J.I.; Williams, R.O. The impact of pulmonary diseases on the fate of inhaled medicines—A review. Int. J. Pharm. 2014, 461, 112–128. [Google Scholar] [CrossRef]
- Hakkimane, S.S.; Shenoy, V.P.; Gaonkar, S.L.; Bairy, I.; Guru, B.R. Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis H37Rv strain. Int. J. Nanomedicine 2018, 13, 4303–4318. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 Dye Encapsulated in cRGD-Conjugated Solid Lipid Nanoparticles for NIR Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef]
- Li, S.; Johnson, J.; Peck, A.; Xie, Q. Near infrared fluorescent imaging of brain tumor with IR780 dye incorporated phospholipid nanoparticles. J. Transl. Med. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Boshra, M.S.; Almeldien, A.G.; Eldin, R.S.; Elberry, A.A.; Abdelwahab, N.S.; Salem, M.N.; Rabea, H.; Abdelrahim, M.E.A. Inhaled salbutamol from aerolizer and diskus at different inhalation flows, inhalation volume and number of inhalations in both healthy subjects and COPD patients. Exp. Lung Res. 2019, 45, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Boshra, M.S.; Almeldien, A.G.; Salah Eldin, R.; Elberry, A.A.; Abdelwahab, N.S.; Nabil Salem, M.; Rabea, H.; Abdelrahim, M.E.A. Total emitted dose of salbutamol sulphate at different inhalation flows and inhalation volumes through different types of dry powder inhalers. Exp. Lung Res. 2018, 44, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Abadelah, M.; Chrystyn, H.; Bagherisadeghi, G.; Abdalla, G.; Larhrib, H. Study of the Emitted Dose After Two Separate Inhalations at Different Inhalation Flow Rates and Volumes and an Assessment of Aerodynamic Characteristics of Indacaterol Onbrez Breezhaler® 150 and 300 μg. AAPS PharmSciTech 2018, 19, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semmler-Behnke, M.; Takenaka, S.; Fertsch, S.; Wenk, A.; Seitz, J.; Mayer, P.; Oberdörster, G.; Kreyling, W.G. Efficient elimination of inhaled nanoparticles from the alveolar region: Evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ. Health Perspect. 2007, 115, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Del Rosal, B.; Villa, I.; Jaque, D.; Sanz-Rodríguez, F. In vivo autofluorescence in the biological windows: The role of pigmentation. J. Biophotonics 2016, 9, 1059–1067. [Google Scholar] [CrossRef]
- Zeidler-Erdely, P.C.; Antonini, J.M.; Meighan, T.G.; Young, S.-H.; Eye, T.J.; Hammer, M.A.; Erdely, A. Comparison of cell counting methods in rodent pulmonary toxicity studies: Automated and manual protocols and considerations for experimental design. Inhal. Toxicol. 2016, 28, 410–420. [Google Scholar] [CrossRef]
- Warheit, D.B.; Hartsky, M.A.; Stefaniak, M.S. Comparative physiology of rodent pulmonary macrophages: In vitro functional responses. J. Appl. Physiol. 1988, 64, 1953–1959. [Google Scholar] [CrossRef]
- Sapru, K.; Stotland, P.K.; Stevenson, M.M. Quantitative and qualitative differences in bronchoalveolar inflammatory cells in Pseudomonas aeruginosa-resistant and -susceptible mice. Clin. Exp. Immunol. 1999, 115, 103–109. [Google Scholar] [CrossRef]
- Crowell, R.E.; Heaphy, E.; Valdez, Y.E.; Mold, C.; Lehnert, B.E. Alveolar and interstitial macrophage populations in the murine lung. Exp. Lung Res. 1992, 18, 435–446. [Google Scholar] [CrossRef]
- Geiser, M.; Serra, A.L.; Cruz-Orive, L.M.; Baumann, M.; Im Hof, V.; Gehr, P. Efficiency of airway macrophage recovery by bronchoalveolar lavage in hamsters: A stereological approach. Eur. Respir. J. 1995, 8, 1712–1718. [Google Scholar] [CrossRef]
- Tan, J.L.; Liu, W.; Nelson, C.M.; Raghavan, S.; Chen, C.S. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004, 10, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Kremer, L.; Halloum, I.; Butani, S. Dry-Powder Inhaler Formulation of Rifampicin: An Improved Targeted Delivery System for Alveolar Tuberculosis. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 388–398. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Q.; Shen, Y.; Lu, G.; Yao, H.; Chen, Y.; Zhou, J. Involvement of mannose receptor in the preventive effects of mannose in lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 2010, 641, 229–237. [Google Scholar] [CrossRef]
- Chieppa, M.; Bianchi, G.; Doni, A.; Del Prete, A.; Sironi, M.; Laskarin, G.; Monti, P.; Piemonti, L.; Biondi, A.; Mantovani, A.; et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 2003, 171, 4552–4560. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tachado, S.D.; Patel, N.; Zhu, J.; Imrich, A.; Manfruelli, P.; Cushion, M.; Kinane, T.B.; Koziel, H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J. Leukoc. Biol. 2005, 78, 665–674. [Google Scholar] [CrossRef]
- Ragusa, J.; Gonzalez, D.; Li, S.; Noriega, S.; Skotak, M.; Larsen, G. Glucosamine/L-lactide copolymers as potential carriers for the development of a sustained rifampicin release system using Mycobacterium smegmatis as a tuberculosis model. Heliyon 2019, 5, e01539. [Google Scholar] [CrossRef] [Green Version]
- Woods, A.; Patel, A.; Spina, D.; Riffo-Vasquez, Y.; Babin-Morgan, A.; de Rosales, R.T.M.; Sunassee, K.; Clark, S.; Collins, H.; Bruce, K.; et al. In vivo biocompatibility, clearance, and biodistribution of albumin vehicles for pulmonary drug delivery. J. Control. Release 2015, 210, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dutta, N.K.; Illei, P.B.; Peloquin, C.A.; Pinn, M.L.; Mdluli, K.E.; Nuermberger, E.L.; Grosset, J.H.; Karakousis, P.C. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob. Agents Chemother. 2012, 56, 3726–3731. [Google Scholar] [CrossRef] [Green Version]
- Nau, R.; Wellmer, A.; Soto, A.; Koch, K.; Schneider, O.; Schmidt, H.; Gerber, J.; Michel, U.; Brück, W. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 1999, 179, 1557–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| SLNas/MS | SLNas/MS-ST | SLNas/ST | SLNas/F127 | |
|---|---|---|---|---|
| Size (nm) | 559 ± 113 | 452 ± 92 | 520 ± 11 | 855 ± 97 |
| PDI | 0.68 ± 0.10 | 0.41 ± 0.02 | 0.56 ± 0.03 | 0.71 ± 0.06 |
| Z-potential (mV) | −43.1 ± 1.6 | −39.4 ± 1.9 | −54.6 ± 2.2 | −8.5 ± 0.3 |
| Drug loading (%, w/w) | 9.6 ± 0.2 | 10.3 ± 0.4 | 11.8 ± 0.4 | 8.10 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, E.; Nascimento, T.L.; Iannuccelli, V.; Costantino, L.; Lima, E.M.; Leo, E.; Siligardi, C.; Gualtieri, M.L.; Maretti, E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials 2020, 10, 568. https://doi.org/10.3390/nano10030568
Truzzi E, Nascimento TL, Iannuccelli V, Costantino L, Lima EM, Leo E, Siligardi C, Gualtieri ML, Maretti E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials. 2020; 10(3):568. https://doi.org/10.3390/nano10030568
Chicago/Turabian StyleTruzzi, Eleonora, Thais Leite Nascimento, Valentina Iannuccelli, Luca Costantino, Eliana Martins Lima, Eliana Leo, Cristina Siligardi, Magdalena Lassinantti Gualtieri, and Eleonora Maretti. 2020. "In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment?" Nanomaterials 10, no. 3: 568. https://doi.org/10.3390/nano10030568
APA StyleTruzzi, E., Nascimento, T. L., Iannuccelli, V., Costantino, L., Lima, E. M., Leo, E., Siligardi, C., Gualtieri, M. L., & Maretti, E. (2020). In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials, 10(3), 568. https://doi.org/10.3390/nano10030568