Reinforcement of Natural Rubber Latex Using Jute Carboxycellulose Nanofibers Extracted Using Nitro-Oxidation Method
Abstract
:1. Introduction
2. Methodologies
2.1. Materials
2.2. Experimental Method
2.2.1. Preparation of Carboxycellulose Nanofibers (NOCNF)
2.2.2. Nanocomposite Preparation
2.2.3. Characterization of Carboxycellulose Nanofibers (NOCNF)
Fourier Transform Infra-Red Spectrometry (FTIR)
Conductometric Titration Method
Lignin and Hemicellulose Analysis in Raw Jute Fibers and NOCNF
Transmission Electron Microscopy (TEM)
Atomic Force Microscopy (AFM)
Zeta Potential Measurements
Dynamic Light Scattering (DLS)
Contact Angle Measurement
Tensile Test
Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Characterization of NOCNF
3.2. Characterization of Natural Rubber Latex (NRL) and Composite Films
3.3. SEM Images
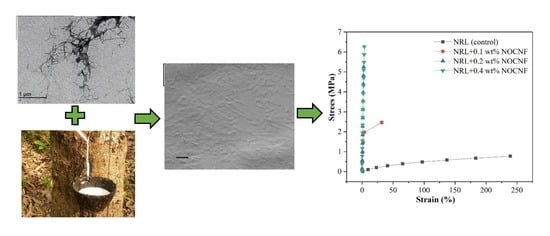
3.4. Mechanical Properties of Latex and Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gangadhar, V.; Babu, S.; Cadambi, R.M.; Rao, A.R.; Venkataram, N. Design and Analysis of Rubber Pallet for Industrial Application. Mater. Today-Proc. 2017, 4, 10886–10893. [Google Scholar] [CrossRef]
- Sasikala, A.; Kala, A. Thermal Stability And Mechanical Strength Analysis of EVA and Blend of EVA With Natural Rubber. Mater. Today-Proc. 2018, 5, 8862–8867. [Google Scholar] [CrossRef]
- Fumagalli, M.; Berriot, J.; de Gaudemaris, B.; Veyland, A.; Putaux, J.L.; Molina-Boisseau, S.; Heux, L. Rubber materials from elastomers and nanocellulose powders: Filler dispersion and mechanical reinforcement. Soft Matter 2018, 14, 2638–2648. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; López, M.D. New application of guayule resin in controlled release formulations. Ind. Crop. Prod. 2013, 43, 44–49. [Google Scholar] [CrossRef]
- Roy, K.; Jatejarungwong, C.; Potiyaraj, P. Development of highly reinforced maleated natural rubber nanocomposites based on sol-gel-derived nano alumina. J. Appl. Polym. Sci. 2018, 135, 46248. [Google Scholar] [CrossRef]
- Fedorko, G.; Molnar, V.; Dovica, M.; Toth, T.; Fabianova, J.; Strohmandl, J.; Neradilova, H.; Hegedus, M.; Belusko, M. Analysis of defects in carcass of rubber-textile conveyor belts using metrotomography. J. Ind. Text. 2018, 47, 1812–1829. [Google Scholar] [CrossRef]
- IRSG. Rubber Industry Report; International Rubber Study Group: Singapore, 2013. [Google Scholar]
- Rasutis, D.; Soratana, K.; McMahan, C.; Landis, A.E. A sustainability review of domestic rubber from the guayule plant. Ind. Crop. Prod. 2015, 70, 383–394. [Google Scholar] [CrossRef]
- Riyajan, S.A.; Patisat, S. A Novel Packaging Film from Cassava Starch and Natural Rubber. J. Polym. Environ. 2018, 26, 2845–2854. [Google Scholar] [CrossRef]
- Kohjiya, S.; Ikeda, Y. Introduction. In Chemistry, Manufacture and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. xvii–xxvi. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Raut, A.; Isseroff, R.; Xue, Y.; Zhou, Y.; Sandhu, B.; Schein, T.; Zeliznyak, T.; Sharma, P.; et al. Operation of proton exchange membrane (PEM) fuel cells using natural cellulose fiber membranes. Sustain. Energy Fuels 2019, 3, 2725–2732. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Hosseinmardi, A.; Annamalai, P.K.; Wang, L.Z.; Martin, D.; Amiralian, N. Reinforcement of natural rubber latex using lignocellulosic nanofibers isolated from spinifex grass. Nanoscale 2017, 9, 9510–9519. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Sanches, A.O.; Medeiros, E.S.; Mattoso, L.H.C.; McMahan, C.M.; Malmonge, J.A. Nanocomposites of natural rubber and polyaniline-modified cellulose nanofibrils. J. Therm. Anal. Calorim. 2014, 117, 387–392. [Google Scholar] [CrossRef]
- Kato, H.; Nakatsubo, F.; Abe, K.; Yano, H. Crosslinking via sulfur vulcanization of natural rubber and cellulose nanofibers incorporating unsaturated fatty acids. RSC Adv. 2015, 5, 29814–29819. [Google Scholar] [CrossRef]
- Pingot, M.; Szadkowski, B.; Zaborski, M. Effect of carbon nanofibers on mechanical and electrical behaviors of acrylonitrile-butadiene rubber composites. Polym. Adv. Technol. 2018, 29, 1661–1669. [Google Scholar] [CrossRef]
- Rashid, E.S.A.; Julkapli, N.B.M.; Yehya, W.A.H. Reinforcement effect of nanocellulose on thermal stability of nitrile butadiene rubber (NBR) composites. J. Appl. Polym. Sci. 2018, 135, 46594. [Google Scholar] [CrossRef]
- Li, X.X.; Cho, U.R. Mechanical Performance and Oil Resistance Behavior of Modified Starch/Cellulose with Silica by Adsorption Method Filled into SBR Rubber Latex. Polym. Korea 2018, 42, 492–497. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Sandmann, G.; Schubert, T.; Klein, D. Effect of oxidized regenerated cellulose/collagen matrix on dermal and epidermal healing and growth factors in an acute wound. Wound Repair Regen. 2005, 13, 324–331. [Google Scholar] [CrossRef]
- Dineen, P. Antibacterial Activity of Oxidized Regenerated Cellulose. Surg. Gynecol. Obstet. 1976, 142, 481–486. [Google Scholar]
- Wu, H.L.; Williams, G.R.; Wu, J.Z.; Wu, J.R.; Niu, S.W.; Li, H.Y.; Wang, H.J.; Zhu, L.M. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; John, M.; Narine, S.S.; Thomas, S.; Anandjiwala, R. Physicomechanical properties of nanocomposites based on cellulose nanofibre and natural rubber latex. Cellulose 2013, 20, 417–427. [Google Scholar] [CrossRef]
- Guo, W.W.; Wang, X.; Zhang, P.; Liu, J.J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Tominaga, Y.; Sato, K.; Hotta, Y.; Shibuya, H.; Sugie, M.; Saruyama, T. Improvement of thermal conductivity of composite film composed of cellulose nanofiber and nanodiamond by optimizing process parameters. Cellulose 2018, 25, 3973–3983. [Google Scholar] [CrossRef]
- Isogai, A. Wood nanocelluloses: Fundamentals and applications as new bio-based nanomaterials. J. Wood Sci. 2013, 59, 449–459. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; de Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s Acid Modified Cellulose Nanofiber-Based Polyvinylidene Fluoride Microfiltration Membrane for Dye Water Treatment and Nanoparticle Removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Zheng, B.; Sharma, S.K.; Zhan, C.; Wang, R.; Bhatia, S.R.; Hsiao, B.S. High Aspect Ratio Carboxycellulose Nanofibers Prepared by Nitro-Oxidation Method and Their Nanopaper Properties. ACS Appl. Nano Mater. 2018, 1, 3969–3980. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Zhan, C.; Sharma, P.R.; Geng, L.; Sharma, S.K.; Wang, R.; Joshi, R.; Hsiao, B.S. Structural characterization of carboxyl cellulose nanofibers extracted from underutilized sources. Sci. China Technol. Sci. 2019, 62, 971–981. [Google Scholar] [CrossRef]
- Kumar, R.; Kumari, S.; Surah, S.S.; Rai, B.; Kumar, R.; Sirohi, S.; Kumar, G. A simple approach for the isolation of cellulose nanofibers from banana fibers. Mater. Res. Express 2019, 6, 105601. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, B.; Kumar, G. A Simple Approach for the Synthesis of Cellulose Nanofiber Reinforced Chitosan/PVP Bio Nanocomposite Film for Packaging. J. Polym. Environ. 2019, 27, 2963–2973. [Google Scholar] [CrossRef]
- Lin, S.-S. Degradation Behaviors of Natural, Guayule, and Synthetic Isoprene Rubbers. Rubber Chem. Technol. 1989, 62, 315–331. [Google Scholar] [CrossRef]
- Geng, L.; Naderi, A.; Mao, Y.; Zhan, C.; Sharma, P.; Peng, X.; Hsiao, B.S. Rheological Properties of Jute-Based Cellulose Nanofibers under Different Ionic Conditions. In Nanocelluloses: Their Preparation, Properties, and Applications; American Chemical Society: Washington, DC, USA, 2017; Volume 1251, pp. 113–132. [Google Scholar]
- Sukhlaaied, W.; Riyajan, S.-A. A Novel Environmentally Compatible Bio-Based Product from Gelatin and Natural Rubber: Physical Properties. J. Polym. Environ. 2018, 26, 2708–2719. [Google Scholar] [CrossRef]
- Venugopal, B.; Gopalakrishnan, J. Reinforcement of natural rubber using cellulose nanofibres isolated from Coconut spathe. Mater. Today-Proc. 2018, 5, 16724–16731. [Google Scholar] [CrossRef]
- Wu, Q.; Meng, Y.; Wang, S.; Li, Y.; Fu, S.; Ma, L.; Harper, D. Rheological behavior of cellulose nanocrystal suspension: Influence of concentration and aspect ratio. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]







| Sample | Carboxylate Content (mmol/g) | Zeta Potential (mV) | Residual Lignin (%) KL/ASL a | Residual hemicellulose (%) | Length/Width (nm) | Thickness (nm) |
|---|---|---|---|---|---|---|
| NOCNF | 0.94 | −115 ± 4 | 0.58/1.36 | 65 | 524 ± 203/ 7 ± 2 | 2.9 |
| Sample | Ym (kPa) | UTS (MPa) | λmax (%) |
|---|---|---|---|
| NRL | 3.3 | 0.77 | 234 |
| NRL 0.1 | 79.6 | 2.5 | 31.4 |
| NRL 0.2 | 2080 | 5.2 | 2.5 |
| NRL 0.4 | 1770 | 6.2 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.K.; Sharma, P.R.; Lin, S.; Chen, H.; Johnson, K.; Wang, R.; Borges, W.; Zhan, C.; Hsiao, B.S. Reinforcement of Natural Rubber Latex Using Jute Carboxycellulose Nanofibers Extracted Using Nitro-Oxidation Method. Nanomaterials 2020, 10, 706. https://doi.org/10.3390/nano10040706
Sharma SK, Sharma PR, Lin S, Chen H, Johnson K, Wang R, Borges W, Zhan C, Hsiao BS. Reinforcement of Natural Rubber Latex Using Jute Carboxycellulose Nanofibers Extracted Using Nitro-Oxidation Method. Nanomaterials. 2020; 10(4):706. https://doi.org/10.3390/nano10040706
Chicago/Turabian StyleSharma, Sunil K., Priyanka R. Sharma, Simon Lin, Hui Chen, Ken Johnson, Ruifu Wang, William Borges, Chengbo Zhan, and Benjamin S. Hsiao. 2020. "Reinforcement of Natural Rubber Latex Using Jute Carboxycellulose Nanofibers Extracted Using Nitro-Oxidation Method" Nanomaterials 10, no. 4: 706. https://doi.org/10.3390/nano10040706
APA StyleSharma, S. K., Sharma, P. R., Lin, S., Chen, H., Johnson, K., Wang, R., Borges, W., Zhan, C., & Hsiao, B. S. (2020). Reinforcement of Natural Rubber Latex Using Jute Carboxycellulose Nanofibers Extracted Using Nitro-Oxidation Method. Nanomaterials, 10(4), 706. https://doi.org/10.3390/nano10040706