Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of IO NPs
2.3. Preparation of HCA-Coated IO NPs
2.4. Preparation of NDPM-Coated IO NPs
2.5. Preparation of MLs
2.6. Hydrodynamic Diameter and Zeta Potential Measurements
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Measurements
2.8. Magnetic Measurements
2.9. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.10. Transmission Electron Microscopy (TEM)
2.11. Nuclear Magnetic Resonance (NMR)
2.12. NMR Relaxivity Measurements
2.13. In Vitro Experiments
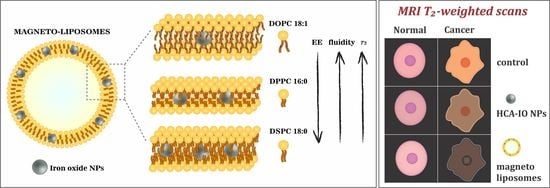
3. Results
3.1. Preparation and Characterization of Nitrodopamine Palmitate (NDPM)-Coated Magnetic Nanoparticles
3.2. MRI Relaxivity Measurements of MLs
3.3. In Vitro MRI Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Gale, E.M.; Caravan, P. MR imaging probes: Design and applications. Dalt. Trans. 2015, 44, 4804. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [PubMed]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar]
- Corot, C.; Robert, P.; Idée, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [Green Version]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory Overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Carvalho, A.; Goncalves, M.C.; Martins, M.B.F.; Meixedo, D.; Feio, G. Relaxivities of magnetoliposomes: The effect of cholesterol. Magn. Reson. Imaging 2013, 31, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Marie, H.; Lemaire, L.; Franconi, F.; Lajnef, S.; Frapart, Y.-M.; Nicolas, V.; Frebourg, G.; Trichet, M.; Menager, C.; Lesieur, S. Superparamagnetic Liposomes for MRI Monitoring and External Magnetic Field-Induced Selective Targeting of Malignant Brain Tumors. Adv. Funct. Mater. 2015, 25, 1258–1269. [Google Scholar] [CrossRef]
- Garnier, B.; Tan, S.; Miraux, S.; Bled, E.; Brisson, A.R. Optimized synthesis of 100 nm diameter magnetoliposomes with high content of maghemite particles and high MRI effect. Contrast Media Mol. Imaging 2012, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.R.; Cruz, M.M.; Gonçalves, M.C.; Carvalho, A.; Feio, G.; Martins, M.B.F. Synthesis and characterization of magnetoliposomes for MRI contrast enhancement. Int. J. Pharm. 2013, 446, 183–190. [Google Scholar] [CrossRef]
- Béalle, G.; Di Corato, R.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clément, O.; Gazeau, F.; Wilhelm, C.; Ménager, C. Ultra magnetic liposomes for MR imaging, targeting, and hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T₂ Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, 1209. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Wang, S.; Zhang, Y.; Gao, J.; Hong, L.; Wang, X.; Wu, W.; Jiang, X. Ultra-high relaxivity iron oxide nanoparticles confined in polymer nanospheres for tumor MR imaging. J. Mater. Chem. B 2015, 5702, 5702–5710. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, X.; Wang, L.; Yang, L.; Mao, H. Improving the magnetic resonance imaging contrast and detection methods with engineered magnetic nanoparticles. Theranostics 2012, 2, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Ferrauto, G.; Delli Castelli, D.; Di Gregorio, E.; Terreno, E.; Aime, S. LipoCEST and cellCEST imaging agents: Opportunities and challenges. WIREs Nanomed. Nanobiotechnol. 2016, 8, 602–618. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Kostevšek, N.; Hudoklin, S.; Kreft, M.E.; Serša, I.; Sepe, A.; Jagličić, Z.; Vidmar, J.; Ščančar, J.; Šturm, S.; Kobe, S.; et al. Magnetic interactions and: In vitro study of biocompatible hydrocaffeic acid-stabilized Fe-Pt clusters as MRI contrast agents. RSC Adv. 2018, 8, 14694–14704. [Google Scholar] [CrossRef] [Green Version]
- Niebel, T.P.; Heiligtag, F.J.; Kind, J.; Zanini, M.; Lauria, A.; Niederberger, M.; Studart, A.R. Multifunctional microparticles with uniform magnetic coatings and tunable surface chemistry. RSC Adv. 2014, 4, 62483–62491. [Google Scholar] [CrossRef]
- Višnjar, T.; Kocbek, P.; Kreft, M.E. Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem. Cell Biol. 2012, 137, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Resnik, N.; Repnik, U.; Kreft, M.E.; Sepčić, K.; Maček, P.; Turk, B.; Veranič, P. Highly Selective Anti-Cancer Activity of Cholesterol-Interacting Agents Methyl-β-Cyclodextrin and Ostreolysin A/Pleurotolysin B Protein Complex on Urothelial Cancer Cells. PLoS ONE 2015, 10, e0137878. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Hudoklin, S.; Sterle, M. Establishment and characterization of primary and subsequent subcultures of normal mouse urothelial cells. Folia Biol. (Praha) 2005, 51, 126–132. [Google Scholar] [PubMed]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Serna, C.J. Magnetic properties of uniform g-Fe2O3 nanoparticles smaller than 5 nm prepared by laser pyrolysis. J. Mater. Res. 1999, 14, 3066–3072. [Google Scholar] [CrossRef]
- Bixner, O.; Lassenberger, A.; Baurecht, D.; Reimhult, E. Complete Exchange of the Hydrophobic Dispersant Shell on Monodisperse Superparamagnetic Iron Oxide Nanoparticles. Langmuir 2015, 31, 9198–9204. [Google Scholar] [CrossRef] [Green Version]
- Smolensky, E.D.; Park, H.Y.E.; Berquó, T.S.; Pierre, V.C. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications - effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [Green Version]
- de Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Morata, L.; Giannotti, M.I.; Sanz, F. Influence of cholesterol on the phase transition of lipid bilayers: A temperature-controlled force spectroscopy study. Langmuir 2012, 28, 12851–12860. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Kostevšek, N.; Abramovič, I.; Hudoklin, S.; Kreft, M.E.; Serša, I.; Sepe, A.; Vidmar, J.; Šturm, S.; Spreitzer, M.; Ščančar, J.; et al. Hybrid FePt/SiO2/Au nanoparticles as a theranostic tool: In vitro photo-thermal treatment and MRI imaging. Nanoscale 2018, 10, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.F.A.; Gordon, R.E. Water in malignant tissue, measured by cell refractometry and nuclear magnetic resonance. J. Microsc. 1982, 128, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Salvatore, A.; Montis, C.; Berti, D.; Baglioni, P. Multifunctional Magnetoliposomes for Sequential Controlled Release. ACS Nano 2016, 10, 7749–7760. [Google Scholar] [CrossRef]
- Chen, Y.; Bose, A.; Bothun, G.D. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 4, 3215–3221. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Ma, J.; Li, Q.; Li, Y.; Zhou, X.; Zhao, D.; Song, H.; Chen, Q.; Zhu, X. Light/magnetic hyperthermia triggered drug released from multi-functional thermo-sensitive magnetoliposomes for precise cancer synergetic theranostics. J. Control. Release 2018, 272, 145–158. [Google Scholar] [CrossRef]
- Scheu, R.; Rankin, B.M.; Chen, Y.; Jena, K.C.; Ben-Amotz, D.; Roke, S. Charge asymmetry at aqueous hydrophobic interfaces and hydration shells. Angew. Chem.-Int. Ed. 2014, 126, 9714–9717. [Google Scholar] [CrossRef]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultrastable Iron Oxide Nanoparticle Colloidal Suspensions Using Dispersants with Catechol-Derived Anchor Groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Pereira, S.; Egbu, R.; Jannati, G.; Al-Jamal, W.T. Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int. J. Pharm. 2016, 514, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Shi, X.; Shen, M. Hydrothermal Synthesis and Functionalization of Iron Oxide Nanoparticles for MR Imaging Applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Li, Z.; Yi, P.W.; Sun, Q.; Lei, H.; Li Zhao, H.; Zhu, Z.H.; Smith, S.C.; Lan, M.B.; Lu, G.Q. Ultrasmall water-soluble and biocompatible magnetic iron oxide nanoparticles as positive and negative dual contrast agents. Adv. Funct. Mater. 2012, 22, 2387–2393. [Google Scholar] [CrossRef]
- Jacques, V.; Dumas, S.; Sun, W.-C.; Troughton, J.; Greenfield, M.T.; Caravan, P. High relaxivity MRI contrast agents part 2: Optimization of inner- and second-sphere relaxivity. Invest. Radiol. 2010, 45, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, K.V.; Merz, K.M.; Gaber, B.P. Structure and Dynamics of the Dilauroylphosphatidylethanolamine Lipid Bilayer. Biochemistry 1992, 31, 7656–7664. [Google Scholar] [CrossRef]
- Carvalho, A.; Martins, M.B.F.; Corvo, M.L.; Feio, G. Enhanced contrast efficiency in MRI by PEGylated magnetoliposomes loaded with PEGylated SPION: Effect of SPION coating and micro-environment. Mater. Sci. Eng. C 2014, 43, 521–526. [Google Scholar] [CrossRef]
- Dumas, S.; Jacques, V.; Sun, W.-C.; Troughton, J.S.; Welch, J.T.; Chasse, J.M.; Schmitt-Willich, H.; Caravan, P. High relaxivity magnetic resonance imaging contrast agents. Part 1. Impact of single donor atom substitution on relaxivity of serum albumin-bound gadolinium complexes. Invest. Radiol. 2010, 45, 600–612. [Google Scholar] [CrossRef] [Green Version]
- Skouras, A.; Mourtas, S.; Markoutsa, E.; De Goltstein, M.-C.; Wallon, C.; Catoen, S.; Antimisiaris, S.G. Magnetoliposomes with high USPIO entrapping efficiency, stability and magnetic properties. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 572–579. [Google Scholar] [CrossRef]
- Shen, S.; Huang, D.; Cao, J.; Chen, Y.; Zhang, X.; Guo, S.; Ma, W.; Qi, X.; Ge, Y.; Wu, L. Magnetic liposomes for light-sensitive drug delivery and combined photothermal–chemotherapy of tumors. J. Mater. Chem. B 2019, 7, 1096–1106. [Google Scholar] [CrossRef]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Commercial Name | NP Size, Coating and Relaxivities (mM−1 s−1) | Intended Use, Status on the Marked |
|---|---|---|
| Endorem in EU/Feridex® IV in USA | 150 nm, dextran-coated IO NPs r1 = 10.1 and r2 = 120 | Primarily designed for liver imaging, approved, and withdrawn from the market due to a lack of users. |
| Resovist® | 60 nm, carbodextran-coated IO NPs r1 = 9.7 and r2 = 189 | Primarily designed for liver imaging, approved, currently available only in Japan. |
| Gastromark® in EU/Lumirem in USA | >300 nm, siloxane-coated IO NPs Relaxivity n.a. | Approved as an oral contrast agent, withdrawn from the market due to a lack of users. |
| Rienso in EU/Faraheme® in USA | 30 nm, carboxyhydrate-coated IO NPs, r1 = 15 and r2 = 89 | Approved for iron-deficiency treatment, withdrawn from EU market, available in US to treat iron-deficiency anaemia in adults with chronic kidney disease, off-label use as MRI contrast agent. |
| Sinerem® in EU/Combidex® in USA | 30 nm, dextran-coated IO NPs r1 = 9.9 and r2 = 65 | Intended for diagnostic use in the characterisation of lymph nodes visualised by MRI, Phase III completed, application was withdrawn in 2007 before approval, failed to demonstrate a statistically significant benefit for sensitivity and specificity. |
| VSOP C184 | Citrate coated 4-8 nm IO NPs, r1 = 14 and r2 = 33.4 | Clinical trials for MR angiography, not approved. |
| Abdoscan® | 3.5 µm Relaxivity n.a. | Oral gastrointestinal imaging, approved in EU but taken off the market in 2000. |
| Siena Plus® | 59 nm IO NPs Relaxivity n.a. | Injected subcutaneously to detect lymph nodes with Sentimag® device, approved in EU. In USA, it is limited to investigational use only. |
| Lipid Formulation | Size (nm) | PDI | ζ-Potential (mV) | Fe (µg/mL) |
|---|---|---|---|---|
| DOPC/DSPE-PEG2000 (96/4) | 144 ± 3 | 0.09 ± 0.01 | −0.6 ± 0.2 | 0.50 ± 0.03 |
| DPPC/DSPE-PEG2000 (96/4) | 150 ± 4 | 0.10 ± 0.04 | 0.8 ± 0.3 | 5.32 ± 0.08 |
| DSPC/DSPE-PEG2000 (96/4) | 135 ± 2 | 0.11± 0.05 | −0.4 ± 0.2 | 15.90 ± 0.10 |
| DOPC/Chol/DSPE-PEG2000 (96/50/4) | 156 ± 4 | 0.10 ± 0.02 | −0.5 ± 0.2 | 0.65 ± 0.05 |
| DPPC/Chol/DSPE-PEG2000 (96/50/4) | 155 ± 6 | 0.11 ± 0.03 | 1.9 ± 0.2 | 1.22 ± 0.08 |
| DSPC/Chol/DSPE-PEG2000 (96/50/4) | 141 ± 5 | 0.07 ± 0.01 | 0.3 ± 0.1 | 1.76 ± 0.08 |
| Sample | r1 (mM−1 s−1) | r2 (mM−1 s−1) |
|---|---|---|
| DOPC/DSPE-PEG2000 (96/4) | <0.5 | 673 ± 12 |
| DPPC/DSPE-PEG2000 (96/4) | <0.5 | 283 ± 9 |
| DSPC/DSPE-PEG2000 (96/4) | <0.5 | 156 ± 4 |
| DOPC/Chol/DSPE-PEG2000 (96/50/4) | <0.5 | 575 ± 5 |
| DPPC/Chol/DSPE-PEG2000 (96/50/4) | 0.6 ± 0.1 | 153 ± 5 |
| DSPC/Chol/DSPE-PEG2000 (96/50/4) | <0.5 | 389 ± 9 |
| HCA-IO NPs | 0.4 ± 0.1 | 16 ± 3 |
| Sample | Total Fe (µg/mL) | Corrected Fe (µg/mL) | Cellular Uptake (%) |
|---|---|---|---|
| NPU control | 0.44 ± 0.06 | - | - |
| NPU + HCA-IO NPs | 0.86 ± 0.05 | 0.42 ± 0.06 | 10.5 ± 1.2% |
| NPU + MLs | 0.64 ± 0.05 | 0.20 ± 0.06 | 5.0 ± 0.6% |
| T24 control | 0.47 ± 0.05 | - | - |
| T24 + HCA-IO NPs | 0.91 ± 0.04 | 0.44 ± 0.05 | 11.0 ± 1.1% |
| T24 + MLs | 0.84 ± 0.05 | 0.38 ± 0.05 | 9.5 ± 0.8% |
| Non-Cholesterol MLs Formulations | Cholesterol-Containing MLs Formulations | |
|---|---|---|
| Fluidity | DOPC > DPPC > DSPC | DOPC/Chol > DSPC/Chol > DPPC/Chol |
| r2 | DOPC > DPPC > DSPC | DOPC/Chol > DSPC/Chol > DPPC/Chol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostevšek, N.; Cheung, C.C.L.; Serša, I.; Kreft, M.E.; Monaco, I.; Comes Franchini, M.; Vidmar, J.; Al-Jamal, W.T. Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations. Nanomaterials 2020, 10, 889. https://doi.org/10.3390/nano10050889
Kostevšek N, Cheung CCL, Serša I, Kreft ME, Monaco I, Comes Franchini M, Vidmar J, Al-Jamal WT. Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations. Nanomaterials. 2020; 10(5):889. https://doi.org/10.3390/nano10050889
Chicago/Turabian StyleKostevšek, Nina, Calvin C. L. Cheung, Igor Serša, Mateja Erdani Kreft, Ilaria Monaco, Mauro Comes Franchini, Janja Vidmar, and Wafa T. Al-Jamal. 2020. "Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations" Nanomaterials 10, no. 5: 889. https://doi.org/10.3390/nano10050889
APA StyleKostevšek, N., Cheung, C. C. L., Serša, I., Kreft, M. E., Monaco, I., Comes Franchini, M., Vidmar, J., & Al-Jamal, W. T. (2020). Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations. Nanomaterials, 10(5), 889. https://doi.org/10.3390/nano10050889