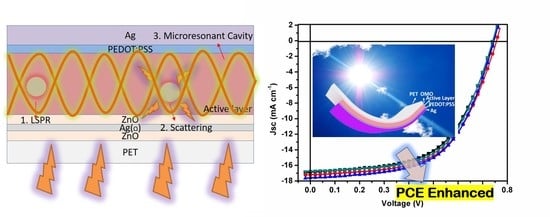
Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells
Abstract
:1. Introduction
2. Experiment Details
2.1. Preparation of OMO Electrodes
2.2. Preparation of Polymer Solar Cell Devices
2.3. Characterizations
2.4. Simulation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, B.; Zhang, D.; Li, M.; Zhong, W.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% efficiency for single-junction organic solar cells. Sci. China Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Meng, L.; Yamin, Z.; Xiangjian, W.; Chenxi, L.; Xin, Z.; Yanbo, W.; Xin, K.; Zuo, X.; Liming, D.; Ruoxi, X. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- An, Q.; Zhang, F.; Zhang, J.; Tang, W.; Deng, Z.; Hu, B. Versatile ternary organic solar cells: A critical review. Energy Environ. Sci. 2016, 9, 281–322. [Google Scholar] [CrossRef]
- Yun, J. Ultrathin Metal films for Transparent Electrodes of Flexible Optoelectronic Devices. Adv. Funct. Mater. 2017, 27, 1606641. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.M.; Chen, S.; Kumari, T.; Kang, S.H.; Cho, Y.; Yang, C. Organic Photovoltaics with Multiple Donor-Acceptor Pairs. Adv. Mater. 2018, 31, 1804762. [Google Scholar] [CrossRef]
- Guo, W.; Xu, Z.; Zhang, F.; Xie, S.; Xu, H.; Liu, X.Y. Recent Development of Transparent Conducting Oxide-Free Flexible Thin-Film Solar Cells. Adv. Funct. Mater. 2016, 26, 8855–8884. [Google Scholar] [CrossRef]
- Zilberberg, K.; Riedl, T. Metal-nanostructures—A modern and powerful platform to create transparent electrodes for thin-film photovoltaics. J. Mater. Chem. A 2016, 4, 14481–14508. [Google Scholar] [CrossRef] [Green Version]
- Chueh, C.C.; Crump, M.; Jen, A.K.Y. Optical Enhancement via Electrode Designs for High-Performance Polymer Solar Cells. Adv. Funct. Mater. 2016, 26, 321–340. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Glowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.-B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwoediauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Palummo, M.; Grossman, J.C. Extraordinary Sunlight Absorption and One Nanometer Thick Photovoltaics Using Two-Dimensional Monolayer Materials. Nano Lett. 2013, 13, 3664–3670. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Zhao, G.; Song, M.; Yu, S.M.; Rha, J.; Shin, J.; Cho, Y.-R.; Yun, J. Simultaneous improvements in self-cleaning and light-trapping abilities of polymer substrates for flexible organic solar cells. J. Mater. Chem. A 2018, 6, 2379–2387. [Google Scholar] [CrossRef]
- Zhao, G.; Kim, S.M.; Lee, S.-G.; Bae, T.-S.; Mun, C.; Lee, S.; Yu, H.; Lee, G.-H.; Lee, H.-S.; Song, M.; et al. Bendable Solar Cells from Stable, Flexible, and Transparent Conducting Electrodes Fabricated Using a Nitrogen-Doped Ultrathin Copper Film. Adv. Funct. Mater. 2016, 26, 4180–4191. [Google Scholar] [CrossRef]
- Wang, W.; Song, M.; Bae, T.-S.; Park, Y.H.; Kang, Y.-C.; Lee, S.-G.; Kim, S.-Y.; Kim, D.H.; Lee, S.; Min, G.; et al. Transparent Ultrathin Oxygen-Doped Silver Electrodes for Flexible Organic Solar Cells. Adv. Funct. Mater. 2014, 24, 1551–1561. [Google Scholar] [CrossRef]
- Behrendt, A.; Friedenberger, C.; Gahlmann, T.; Trost, S.; Becker, T.; Zilberberg, K.; Polywka, A.; Görrn, P.; Riedl, T. Highly Robust Transparent and Conductive Gas Diffusion Barriers Based on Tin Oxide. Adv. Mater. 2015, 27, 5961. [Google Scholar] [CrossRef]
- Yue, B.L.; Jia, D.; Tang, J.; Zhang, A.; Liu, F.; Chen, T.; Barrow, C.; Yang, W.; Liu, J. Improving the rate capability of ultrathin NiCo-LDH nanoflakes and FeOOH nanosheets on surface electrochemically modified graphite fibers for flexible asymmetric supercapacitors. J. Colloid Interface Sci. 2020, 560, 237–246. [Google Scholar] [CrossRef]
- Guo, J.; Tang, J.; Wang, J.; Mao, S.; Li, H.; Wang, Y.; Liu, J.; Wang, Y.; Huang, L.; Kipper, M.J.; et al. Europium(III)-induced water-soluble nano-aggregates of hyaluronic acid and chitosan: Structure and fluorescence. MRS Commun. 2018, 8, 1224–1229. [Google Scholar] [CrossRef]
- Xue, J.; Liu, J.; Mao, S.; Wang, Y.; Shen, W.; Wang, W.; Huang, L.; Li, H.; Tang, J. Recent progress in synthetic methods and applications in solar cells of Ag2S quantum dots. Mater. Res. Bull. 2018, 106, 113–123. [Google Scholar] [CrossRef]
- Yan, P.; Tang, J.; Zhang, Y.; Shen, W.; Wang, Y.; Hou, L.; Tian, R.; Wang, Y.; Li, H.; Huang, L.; et al. SPR-enhanced fluorescence and protein-improved blood compatibility of quadruple core/shell nanostructure of Ag@SiO2@Eu3+(tta)(3)Phen@Protein. Micro Nano Lett. 2018, 13, 1447–1452. [Google Scholar] [CrossRef]
- Yun, J.; Wang, W.; Bae, T.S.; Park, Y.H.; Kang, Y.C.; Kim, D.H.; Lee, S.; Lee, G.H.; Song, M.; Kang, J.W. Preparation of flexible organic solar cells with highly conductive and transparent metal-oxide multilayer electrodes based on silver oxide. ACS Appl. Mater. Interfaces 2013, 5, 9933–9941. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-W.; Lee, J.; Jo, J.; Cho, C.; Lee, J.-Y. Highly Efficient (>10%) Flexible Organic Solar Cells on PEDOT-Free and ITO-Free Transparent Electrodes. Adv. Mater. 2019, 31, 1902447. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ren, X.; Ouyang, D.; Choy, W.C.H. Emerging Novel Metal Electrodes for Photovoltaic Applications. Small 2018, 14, 1703140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sanyoto, B.; Park, W.-T.; Kim, S.; Mandal, S.; Lim, J.-C.; Noh, Y.-Y.; Kim, J.-H. Purification of PEDOT: PSS by Ultrafiltration for Highly Conductive Transparent Electrode of All-Printed Organic Devices. Adv. Mater. 2016, 28, 10149–10154. [Google Scholar] [CrossRef] [PubMed]
- Kan, Z.; Wang, Z.; Firdaus, Y.; Babics, M.; Alshareef, H.N.; Beaujuge, P.M. Atomic-layer-deposited AZO outperforms ITO in high-efficiency polymer solar cells. J. Mater. Chem. A 2018, 6, 10176–10183. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Chen, X.; Wei, J.; Ji, G.; Wang, C.; Zhao, W.; Lai, J.; Zha, W.; Li, Z.; Yan, L.; et al. Efficiency above 12% for 1 cm(2) Flexible Organic Solar Cells with Ag/Cu Grid Transparent Conducting Electrode. Adv. Sci. 2019, 6, 1901490. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.H.; Lee, D.H.; Jo, J.; Kim, S.Y.; Yoo, J.-W.; Kwon, S.-Y. Highly Conductive and Environmentally Stable Organic Transparent Electrodes Laminated with Graphene. Adv. Funct. Mater. 2016, 26, 7234–7243. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, D.; Gu, D.; Kim, H.; Ling, T.; Wu, Y.-K.R.; Guo, L.J. An Ultrathin, Smooth, and Low-Loss Al-Doped Ag Film and Its Application as a Transparent Electrode in Organic Photovoltaics. Adv. Mater. 2014, 26, 5696–5701. [Google Scholar] [CrossRef] [Green Version]
- Hutter, O.S.; Stec, H.M.; Hatton, R.A. An Indium-Free Low Work Function Window Electrode for Organic Photovoltaics Which Improves with In-Situ Oxidation. Adv. Mater. 2013, 25, 284–288. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Omrani, M.K.; Fallah, H. Improving light trapping of polymer solar cell via doping a new array of triple core-shell spherical nanoparticles utilizing realistic modeling. Sol. Energy 2018, 163, 600–609. [Google Scholar] [CrossRef]
- Ou, Q.D.; Li, Y.Q.; Tang, J.X. Light Manipulation in Organic Photovoltaics. Adv. Sci. (Weinheim) 2016, 3, 1600123. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Samajdar, D.P.; Sharma, D. Plasmonic effect of different nanoarchitectures in the efficiency enhancement of polymer based solar cells: A review. Sol. Energy 2018, 173, 905–919. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.-Y.; Kim, C.; Kim, J.-J. Enhancement of the short circuit current in organic photovoltaic devices with microcavity structures. Appl. Phys. Lett. 2010, 97, 083306. [Google Scholar] [CrossRef]
- Lin, H.W.; Chiu, S.W.; Lin, L.Y.; Hung, Z.Y.; Chen, Y.H.; Lin, F.; Wong, K.T. Device engineering for highly efficient top-illuminated organic solar cells with microcavity structures. Adv. Mater. 2012, 24, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, C.W.; Huang, Z.Y.; Lin, W.C.; Lin, L.Y.; Lin, F.; Wong, K.T.; Lin, H.W. Microcavity-embedded, colour-tuneable, transparent organic solar cells. Adv. Mater. 2014, 26, 1129–1134. [Google Scholar] [CrossRef]
- Yao, K.; Xin, X.-K.; Chueh, C.-C.; Chen, K.-S.; Xu, Y.-X.; Jen, A.K.Y. Enhanced Light-Harvesting by Integrating Synergetic Microcavity and Plasmonic Effects for High-Performance ITO-Free Flexible Polymer Solar Cells. Adv. Funct. Mater. 2015, 25, 567–574. [Google Scholar] [CrossRef]
- Yao, K.; Salvador, M.; Chueh, C.-C.; Xin, X.-K.; Xu, Y.-X.; deQuilettes, D.W.; Hu, T.; Chen, Y.; Ginger, D.S.; Jen, A.K.Y. A General Route to Enhance Polymer Solar Cell Performance using Plasmonic Nanoprisms. Adv. Energy Mater. 2014, 4, 1400206–1400213. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Miyazaki, H.T. Metal-insulator-metal plasmon nanocavities: Analysis of optical properties. Phys. Rev. B 2007, 75, 035411. [Google Scholar] [CrossRef]
- Shen, W.; Tang, J.; Ning, W.; Yao, W.; Jixian, L.; Linjun, H.; Jiuxing, W.; Yanxin, W.; Wei, W.; Renqiang, Y.; et al. The Improved Efficiency of Polymer Solar Cells by Fluorine Atoms at Ortho-Position of Alkxoyphenyl Group in Benzodithiophene (BDT) Units. Int. J. Electrochem. Sci. 2017, 12, 6676–6693. [Google Scholar] [CrossRef]
- Chen, W.; Huang, G.; Li, X.; Li, Y.; Wang, H.; Jiang, H.; Zhao, Z.; Yu, D.; Wang, E.; Yang, R. Revealing the Position Effect of an Alkylthio Side Chain in Phenyl-Substituted Benzodithiophene-Based Donor Polymers on the Photovoltaic Performance of Non-Fullerene Organic Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 33173–33178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, W.; Bae, T.S.; Lee, S.G.; Mun, C.; Lee, S.; Yu, H.; Lee, G.H.; Song, M.; Yun, J. Stable ultrathin partially oxidized copper film electrode for highly efficient flexible solar cells. Nat. Commun. 2015, 6, 8830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Shen, W.; Tang, J.; Wang, Y.; Liu, J.; Wang, X.; Yang, R.; Snow, C.D.; Huang, L.; Jiao, J.; et al. Enhancing the Power Conversion Efficiency for Polymer Solar Cells by Incorporating Luminescent Nanosolid Micelles as Light Converter. ACS Appl. Energy Mater. 2018, 1, 1445–1454. [Google Scholar] [CrossRef]
- Bu, F.; Shen, W.; Zhang, X.; Wang, Y.; Belfiore, L.A.; Tang, J. Hybrid ZnO Electron Transport Layer by Down Conversion Complexes for Dual Improvements of Photovoltaic and Stable Performances in Polymer Solar Cells. Nanomaterials 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.; Liang, Z.; Wang, N.; Tong, J.; Yang, C.; Bao, X.; Xia, Y. Enhanced Organic Photovoltaic Performance through Modulating Vertical Composition Distribution and Promoting Crystallinity of the Photoactive Layer by Diphenyl Sulfide Additives. ACS Appl. Mater. Interfaces 2019, 11, 7022–7029. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Wang, Y.; Li, H.; Tong, J.; Bao, X.; Xia, Y. Enhanced efficiency of polymer solar cells through synergistic optimization of mobility and tuning donor alloys by adding high-mobility conjugated polymers. J. Mater. Chem. C 2018, 6, 11015–11022. [Google Scholar] [CrossRef]
- Shen, W.; Tang, J.; Wang, Y.; Liu, J.; Huang, L.; Chen, W.; Yang, L.; Wang, W.; Wang, Y.; Yang, R.; et al. Strong Enhancement of Photoelectric Conversion Efficiency of Co-hybridized Polymer Solar Cell by Silver Nanoplates and Core-Shell Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5358–5365. [Google Scholar] [CrossRef]
- Hedley, G.J.; Ruseckas, A.; Samuel, I.D.W. Light Harvesting for Organic Photovoltaics. Chem. Rev. 2017, 117, 796–837. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Wang, W.; Kim, S.M.; Bae, T.-S.; Lee, S.; Kim, D.; Lee, G.-H.; Lee, H.-S.; Song, M. Light trapping in bendable organic solar cells using silica nanoparticle arrays. Energy Environ. Sci. 2015, 8, 932–940. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, W.; Jeong, E.; Lee, S.-G.; Yu, S.M.; Bae, T.-S.; Lee, G.-H.; Han, S.Z.; Tang, J.; Choi, E.-A.; et al. Ultrathin Silver Film Electrodes with Ultralow Optical and Electrical Losses for Flexible Organic Photovoltaics. ACS Appl. Mater. Interfaces 2018, 10, 27510–27520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Li, T.; Wang, J.; Liu, K.; Jiang, Q.; Tang, J.; Zhan, X. Black Phosphorous Quantum Dots Sandwiched Organic Solar Cells. Small 2019, 15, 1903977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, Z.; Wang, H.; Qiao, S.; Liu, Z.; Jiang, H.; Chen, W.; Yang, R. Fluorinated D1(0.5)–A–D2(0.5)–A model terpolymer: Ultrafast charge separation kinetics and electron transfer at the fluorinated D/A interface for power conversion. J. Mater. Chem. A 2020, 8, 1360–1367. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, W.; Jeong, E.; Lee, S.-G.; Chung, H.-S.; Bae, T.-S.; Bae, J.-S.; Lee, G.-H.; Tang, J.; Yun, J. Nitrogen-Mediated Growth of Silver Nanocrystals to Form UltraThin, High-Purity Silver-Film Electrodes with Broad band Transparency for Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 40901–40910. [Google Scholar] [CrossRef] [PubMed]








| TE Type | Ag(O) Thickness (nm) | Average T (%) in 400–800 nm | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) | Calculated Jsc from EQE (mA cm−2) | Rs (Ω cm2) |
|---|---|---|---|---|---|---|---|---|
| ZnO/Ag(O)/ZnO | 6.0 | 92.90 | 0.77 ± 0.01 | 17.62 ± 0.25 | 56.42 ± 0.56 | 7.65 ± 0.11 | 17.45 | 6.57 |
| ZnO/Ag(O)/ZnO | 7.5 | 93.67 | 0.76 ± 0.01 | 17.12 ± 0.32 | 59.96 ± 0.42 | 7.79 ± 0.13 | 17.04 | 3.07 |
| ZnO/Ag(O)/ZnO | 9.0 | 92.96 | 0.76 ± 0.01 | 16.64 ± 0.37 | 58.49 ± 0.63 | 7.40 ± 0.16 | 16.26 | 3.58 |
| ZnO/Ag(O)/ZnO | 12 | 83.60 | 0.76 ± 0.01 | 14.56 ± 0.42 | 56.70 ± 0.78 | 6.27 ± 0.21 | 13.59 | 5.79 |
| ZnO/Ag(O)/ZnO | 15 | 72.79 | 0.77 ± 0.01 | 12.07 ± 0.39 | 50.34 ± 0.69 | 4.65 ± 0.19 | 11.51 | 10.11 |
| ITO/ZnO | 0 | 85.66 | 0.77 ± 0.01 | 13.70 ± 0.22 | 55.13 ± 0.53 | 5.90 ± 0.24 | 9.24 |
| Ag @SiO2 conc. | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) | Rs (Ω cm2) |
|---|---|---|---|---|---|
| 2.0 wt% | 0.76 ± 0.01 | 17.25 ± 0.24 | 55.41 ± 0.71 | 7.26 ± 0.19 | 9.67 |
| 1.5 wt% | 0.76 ± 0.01 | 17.39 ± 0.36 | 56.88 ± 0.84 | 7.51 ± 0.31 | 6.34 |
| 1.0 wt% | 0.77 ± 0.01 | 17.98 ± 0.28 | 58.40 ± 0.52 | 8.04 ± 0.21 | 3.27 |
| 0.5 wt% | 0.76 ± 0.01 | 17.22 ± 0.29 | 58.49 ± 0.44 | 7.69 ± 0.16 | 4.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Zhao, G.; Zhang, X.; Bu, F.; Yun, J.; Tang, J. Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells. Nanomaterials 2020, 10, 944. https://doi.org/10.3390/nano10050944
Shen W, Zhao G, Zhang X, Bu F, Yun J, Tang J. Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells. Nanomaterials. 2020; 10(5):944. https://doi.org/10.3390/nano10050944
Chicago/Turabian StyleShen, Wenfei, Guoqing Zhao, Xiaolin Zhang, Fanchen Bu, Jungheum Yun, and Jianguo Tang. 2020. "Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells" Nanomaterials 10, no. 5: 944. https://doi.org/10.3390/nano10050944
APA StyleShen, W., Zhao, G., Zhang, X., Bu, F., Yun, J., & Tang, J. (2020). Using Dual Microresonant Cavity and Plasmonic Effects to Enhance the Photovoltaic Efficiency of Flexible Polymer Solar Cells. Nanomaterials, 10(5), 944. https://doi.org/10.3390/nano10050944