Study on the Effects of a π Electron Conjugated Structure in Binuclear Metallophthalocyanines Graphene-Based Oxygen Reduction Reaction Catalysts
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Synthesis
2.3.1. Polystyrene Sodium Sulfonate Modified Graphene (PSS-Gr) Preparation
2.3.2. Preparation of Fe2Pc2(EP)4/PSS-Gr
2.4. Evaluation of the Electrocatalytic Activity
3. Results and Discussion
3.1. Morphology and Structure of M2Pc2(EP)4/PSS-Gr Composites
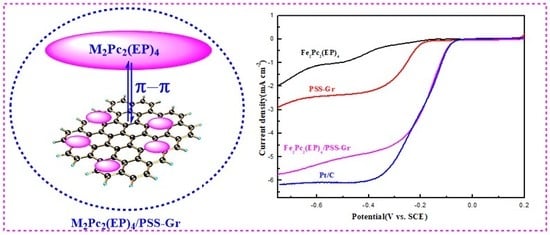
3.2. Effects of π Electron Conjugated Structure for ORR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Jiang, Z.Q.; Wang, X.B.; Ying, W.; Chen, D.; Liu, S.H. Zwitterion threaded metal–organic framework membranes for direct methanol fuel cells. J. Mater. Chem. A 2018, 6, 19547–19554. [Google Scholar] [CrossRef]
- Gago, A.S.; Acosta, D.M.; Arriaga, L.G.; Vante, N.A. Carbon supported ruthenium chalcogenide as cathode catalyst in a microfluidic formic acid fuel cell. J. Power. Sources 2011, 196, 1324–1328. [Google Scholar] [CrossRef]
- Gong, L.Y.; Yang, Z.Y.; Li, K.; Xing, W.; Liu, C.P.; Ge, J.J. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell. J. Energy. Chem. 2018, 27, 1618–1628. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.T.; Hor, T.A.; Zong, Y.; Xiao, P. Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS. Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kim, Y.; Park, J.; Higgins, D.; Shen, S.J.; Schindler, P. Extending the limits of Pt/c catalysts with passivation-gas-incorporated atomic layer deposition. Nat. Cat. 2018, 1, 624–630. [Google Scholar] [CrossRef]
- Rosli, N.F.; Carmen, C.; Martinez, M.; Latiff, N.M. Layered PtTe2 Matches Electrocatalytic Performance of Pt/C for Oxygen Reduction Reaction with Significantly Lower Toxicity. ACS. Sustain. Chem. Eng. 2018, 6, 7432–7441. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2018, 104, 4271–4302. [Google Scholar] [CrossRef]
- Morozan, A.; Campidelli, S.; Filoramo, A.; Jousselme, B.; Palacin, S. Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 2011, 49, 4839–4847. [Google Scholar] [CrossRef]
- Özer, L.M.; Altındal, A.; Özkaya, A.R.; Salih, B.; Bekaroglu, Ö. Synthesis, characterization, OFET and electrochemical properties of novel dimeric metallophthalocyanines. Dalton. Trans. 2013, 42, 6633–6644. [Google Scholar] [CrossRef]
- Zhang, R.L.; Wang, J.; Xu, B.; Huang, X.Y.; Xu, Z.; Zhao, J.S. Investigation of binuclear metal phthalocyanines as electrocatalysts for Li/SOCl2 battery. J. Electrochem. Soc. 2012, 159, H704–H710. [Google Scholar] [CrossRef]
- Moraes, F.C.; Cabral, M.F.; Machado, S.A.; Mascaro, L.H. Electrocatalytic Behavior of Glassy Carbon Electrodes Modified with Multiwalled Carbon Nanotubes and Cobalt Phthalocyanine for Selective Analysis of Dopamine in Presence of Ascorbic Acid. Electroanalysis 2008, 20, 851–857. [Google Scholar] [CrossRef]
- Odabaş, Z.; Altındal, A.; Özkaya, A.R.; Salih, B.; Bekaroglu, Ö. Novel ball-type homo-and hetero-binuclear phthalocyanines with four 1, 10- methylenedinaphthalen-2-ol bridges: Synthesis and characterization, electrical and gas sensing properties and electrocatalytic performance towards oxygen reduction. Sens. Actuators B Chem. 2010, 145, 355–366. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Panomsuwan, G.; Li, O.L.; Saito, N. Heterocarbon nanosheets incorporating iron phthalocyanine for oxygen reduction reaction in both alkaline and acidic media. Phys. Chem. Chem. Phys. 2016, 18, 10856–10863. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.F.; Li, Z.F.; Wang, S. Planar polyphthalocyanine cobalt absorbed on carbon black as stable electrocatalysts for direct methanol fuel cell. J. Power. Sources 2010, 195, 4731–4735. [Google Scholar] [CrossRef]
- Chen, J.M.; Zou, K.Y.; Ding, P.; Deng, J. Conjugated Cobalt Polyphthalocyanine as the Elastic and Reprocessable Catalyst for Flexible Li-CO2 Batteries. Adv. Mater. 2018, 31, 1805484. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Y.; Cai, Z.F.; Wang, D. Single molecular imaging of Iron-Phthalocyanine catalyzed oxygen reduction reaction by in situ scanning tunneling microscopy. ACS Nano 2016, 10, 8746–8750. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Kang, L.; Wang, R.Y.; Duan, X.T.; Liu, Q.Q.; Zhang, R.L. Electrochemical Effects of Lithium-Thionyl Chloride Battery by Central Metal Ions of Phthalocyanines-Tetraacetamide Complexes. J. Electrochem. Soc. 2017, 164, A3628–A3632. [Google Scholar] [CrossRef]
- Peng, Y.X.; Li, Z.P.; Xia, D.G.; Zheng, L.R.; Liao, Y.; Li, K. Probing the influence of the center atom coordination structure in iron phthalocyanine multi-walled carbon nanotube-based oxygen reduction reaction catalysts by X-ray absorption fine structure spectroscopy. J. Power. Sources 2015, 291, 20–28. [Google Scholar] [CrossRef]
- Li, T.; Peng, Y.; Li, K.; Zhang, R.; Zheng, L.; Xia, D. Enhanced activity and stability of binuclear iron (III) phthalocyanine on graphene nanosheets for electrocatalytic oxygen reduction in acid. J. Power. Sources 2015, 293, 511–518. [Google Scholar] [CrossRef]
- Yasemin, C.; Emel, E.; Fatih, D.; Ali, R.Ö.; Bekir, S.; Özer, B. Synthesis, characterization, electrochemistry and VOC sensingproperties of novel ball-type dinuclear metallophthalocyanines. Sens. Actuators B 2014, 202, 1137–1147. [Google Scholar]
- Kakı, E.; Altındal, A.; Salih, B.; Bekaroğlu, Ö. Synthesis, characterization and gas sensing properties of novel homo and hetero binuclear ball-type phthalocyanines. Dalton. Trans. 2015, 44, 8293–8299. [Google Scholar]
- Tolbin, A.Y.; Ivanov, A.V.; Tomilova, L.G.; Zefirov, N.S. Synthesis of 1,2-bis(3,4-dicyanophenoxymethyl)benzene and binuclear zinc phthalocyaninesof clamshell and ball types. J. Porphyr. Phthalocyan 2003, 7, 162–166. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Ivanov, A.V.; Tomilova, L.G.; Zefirov, N.S. Preparation of 1,2-bis(3,4-dicyanophenoxymethyl)benzene and the binuclear zinc phthalocyaninederived from it. Mendeleev. Commun. 2002, 12, 96–97. [Google Scholar] [CrossRef]
- Bekaro˘glu, Ö.; Jiang, J. Functional Phthalocyanine Molecular Materials; Springer: Berlin/Heidelberg, Germany, 2010; Volume 135, pp. 105–136. [Google Scholar]
- Git, N.K.; Özen, Ü.E.; Özer, M.; Salih, B.; Özkaya, A.R.; Glu, Ö.B. Electrocatalytic Activity of Novel Ball-Type Metallophthalocyanines with Trifluoro Methyl Linkages in Oxygen Reduction Reaction and Application as Zn-Air Battery Cathode Catalyst. Electrochim. Acta 2017, 233, 237–248. [Google Scholar]
- Beck, F.; Dammert, W.; Beiss, J.; Hiller, H.; Tolster, R. Electrocatalysis of oxygen cathode by metal-phthalocyanine and metal-dibenzotetraazaannulene. Z. Naturforsch. 1973, 28, 1009–1021. [Google Scholar]
- Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bioelectrochemical systems. J. Power. Sources. 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Liu, X.M.; Xu, T.; Li, Y.L.; Zang, Z.G.; Peng, X.S.; Wei, H.Y. Enhanced X-ray photon response in solution-synthesized CsPbBr3 nanoparticles wrapped by reduced graphene oxide. Sol. Energy Mater. Sol. Cells 2018, 187, 249–254. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, K.; Yan, Y.; Cai, Z.H.; Lin, S.X.; Hu, X.B. Graphene Aerogels Enhanced Phase Change Materials prepared by one-pot method with high thermal conductivity and large latent energy storage. Sol. Energy Mater. Sol. Cells 2018, 185, 487–493. [Google Scholar] [CrossRef]
- Kusuma, J.; Balakrishna, R.G.; Patil, S.; Jyothi, M.S.; Chandan, H.R.; Shwetharani, R. Exploration of Graphene oxide nanoribbons as excellent electron conducting network for third generation solar cells. Sol. Energy Mater. Sol. Cells 2018, 183, 211–219. [Google Scholar] [CrossRef]
- Dai, L.M.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Cui, L.; Lv, G.; Dou, Z. Fabrication of iron phthalocyanine/Graphene micro/nanocomposite by solvothermally assisted π–π assembling method and its application for oxygen reduction reaction. Electrochim. Acta 2013, 106, 272–278. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Tang, S.; Feng, W. Preparation of a Graphene oxide–phthalocyanine hybrid through strong π–π interactions. Carbon 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, B.L.; Zhou, H.W.; Yang, Y.; Chen, W.X.; Zhao, J.S. Graphene Wrapped Phthalocyanine: Enhanced Oxidative Desulfurization for Dibenzothiophene in Fuel. Appl. Organometallic. Chem. 2018, 32, a4477. [Google Scholar] [CrossRef]
- Liu, D.; Peng, J.H.; Li, Z.Y.; Liu, B.; Wang, L. Improvement in the mechanical properties, proton conductivity, and methanol resistance of highly branched sulfonated poly(arylene ether)/Graphene oxide Grafted with flexible alkylsulfonated side chains nanocomposite membranes. J. Power. Sources 2018, 378, 451–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.K.; Zhao, W.; Li, X.M.; Yin, R.; Qian, L. Iron (II) phthalocyanine nanoclusters - Graphene sandwich composite for oxygen reduction reaction catalysts. Mater. Des. 2017, 130, 366–372. [Google Scholar] [CrossRef]
- Cao, R.G.; Thapa, R.; Kim, H.; Xu, X.D.; Kim, M.G.; Li, Q. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, P.; Sampsa, K.; Hämäläinen, M.; Harju, A. Self-Assembly and Orbital Imaging of Metal Phthalocyanines on a Graphene Model Surface. J. Phys. Chem. C 2014, 118, 13320–13325. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Andersen, N.I. Original Mechanochemical Synthesis of Non-Platinum Group Metals Oxygen Reduction Reaction Catalysts Assisted by Sacrificial Support Method. Electrochim. Acta 2015, 179, 154–160. [Google Scholar] [CrossRef]
- Jaouen, F.; Herranz, J.; Lefèvre, M. Cross-Laboratory Experimental Study of Non-Noble-Metal Electrocatalysts for the Oxygen Reduction Reaction. ACS. Appl. Mater. Interfaces 2009, 1, 1623–1639. [Google Scholar] [CrossRef]
- Jin, H.G.; Jiang, X.; Irina, A.; Clair, S.; Valérie, M.; Chendo, C. Microwave-mediated synthesis of bulky lanthanide porphyrin–phthalocyanine triple-deckers: Electrochemical and magnetic properties. ACS Inorg. Chem. 2017, 6, b03056. [Google Scholar] [CrossRef] [Green Version]
- Tejerina, L.; Nazeeruddin, M.K.; GRätzel, M.; Torres, T. Role of the bulky aryloxy Group at the non-peripheral position of phthalocyanines for dye sensitized solar cells. ChemPlusChem 2017, 82, 132–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Li, H.; Chu, D. Unraveling Oxygen Reduction Reaction Mechanisms on Carbon-Supported Fe-Phthalocyanine and Co-Phthalocyanine Catalysts in Alkaline Solutions. J. Phys. Chem. C 2009, 113, 20689–20697. [Google Scholar] [CrossRef]
- Guo, J.; He, H.; Chu, D. OH-Binding Effects on Metallophthalocyanine Catalysts for O2, Reduction Reaction in Anion Exchange Membrane Fuel Cells. Electrocatalysis 2012, 3, 252–264. [Google Scholar] [CrossRef]
- Oberst, J.L.; Thorum, M.S.; Gewirth, A.A. Effect of pH and Azide on the Oxygen Reduction Reaction with a Pyrolyzed Fe Phthalocyanine Catalyst. J. Phys. Chem. C 2018, 116, 25257–25261. [Google Scholar] [CrossRef]
- Özen, Ü.E.; Elvan, D.; Özer, M.; Glu, Ö.B.; Özkaya, A.R. Communication—High-Performance and Non-Precious Bifunctional Oxygen Electrocatalysis with Binuclear Ball-Type Phthalocyanine Based Complexes for Zinc-Air Batteries. J. Electrochem. Soc. 2016, 163, A2001–A2003. [Google Scholar] [CrossRef]
- Tanaka, A.; Fierro, C.; Scherson, D.; Yaeger, E. Electrocatalytic aspects of iron phthalocyanine and its. mu.-oxo derivatives dispersed on high surface area carbon. J. Phys. Chem. 1987, 91, 3799–3807. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Liu, B.; Zhang, Y.; Li, T.; Chen, W.; Zhao, W. Study on the Effects of a π Electron Conjugated Structure in Binuclear Metallophthalocyanines Graphene-Based Oxygen Reduction Reaction Catalysts. Nanomaterials 2020, 10, 946. https://doi.org/10.3390/nano10050946
Zhang G, Liu B, Zhang Y, Li T, Chen W, Zhao W. Study on the Effects of a π Electron Conjugated Structure in Binuclear Metallophthalocyanines Graphene-Based Oxygen Reduction Reaction Catalysts. Nanomaterials. 2020; 10(5):946. https://doi.org/10.3390/nano10050946
Chicago/Turabian StyleZhang, Gai, Bulei Liu, Yufan Zhang, Tiantian Li, Weixing Chen, and Weifeng Zhao. 2020. "Study on the Effects of a π Electron Conjugated Structure in Binuclear Metallophthalocyanines Graphene-Based Oxygen Reduction Reaction Catalysts" Nanomaterials 10, no. 5: 946. https://doi.org/10.3390/nano10050946
APA StyleZhang, G., Liu, B., Zhang, Y., Li, T., Chen, W., & Zhao, W. (2020). Study on the Effects of a π Electron Conjugated Structure in Binuclear Metallophthalocyanines Graphene-Based Oxygen Reduction Reaction Catalysts. Nanomaterials, 10(5), 946. https://doi.org/10.3390/nano10050946