Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Apparatus
2.2. Synthesis of Mn:ZnS QDs
2.3. QDs Based Photocatalytic Experiments for NOFX Degradation
3. Results and Discussion
3.1. Characterization of QDs
3.2. Photocatalytic Degradation of NOFX Using Mn:ZnSQDs
3.2.1. Effect of the Initial pH of NOFX Solution
3.2.2. Effect of Catalyst Loading
3.2.3. Effect of the Initial Concentration of Drug
3.2.4. Effect of Mn2+ Dopant Concentration
3.3. Kinetic Studies
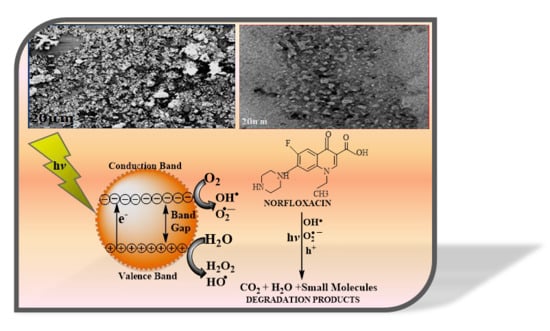
3.4. Probable Photocatalytic Degradation Mechanism of the Photocatalyst
3.5. Role of Active Oxidation Species
3.6. Identification of Transformation Products
3.7. Reusability of Photocatalyst for Degradation of NOFX
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Duong, H.A.; Pham, N.H.; Nguyen, H.T.; Hoang, T.T.; Pham, H.V.; Pham, V.C.; Berg, M.; Giger, W.; Alder, A.C. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere 2008, 72, 968–973. [Google Scholar] [CrossRef]
- Hu, B.; Cai, F.; Chen, T. Hydrothermal synthesis g-C3N4/Nano-InVO4 nanocomposites and enhanced photocatalytic activity for hydrogen production under visible light irradiation. ACS Appl. Mater. Interf. 2015, 7, 18247–18256. [Google Scholar] [CrossRef] [PubMed]
- Trovo, A.G.; Nigeria, R.F.; Aguera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process-chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Bi, E.; Chen, H. Sorption Behavior of Ofloxacin to Kaolinite: Effects of pH, Ionic Strength, and Cu(II). Water Air Soil Pollut. 2017, 228, 46. [Google Scholar] [CrossRef]
- Kong, D.; Liang, B.; Yun, H.; Cheng, H.; Ma, J.; Cui, M.; Wang, A.; Ren, N. Cathodic degradation of antibiotics: Characterization and pathway analysis. Water Res. 2015, 72, 281–292. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.R.; Gao, W.; Jia, Z.; Carvalho, M.F. Biodegradation of the veterinary antibiotics enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581, 582359–582368. [Google Scholar] [CrossRef]
- Zwiener, C.; Glauner, T.; Frimmel, F.H. Biodegradation of Pharmaceutical Residues Investigated by SPE-GC/ITD-MS and On-Line Derivatization. J. High Resolut. Chromatogr. 2000, 23, 474–478. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment--a literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Elimelech, M. Pharmaceutical retention mechanisms by nanofiltration membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Hartig, C.; Ernst, M.; Jekel, M. Membrane filtration of two sulphonamides in tertiary effluents and subsequent adsorption on activated carbon. Water Res. 2001, 35, 3998–4003. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.A.; Wert, E. Fate of Endocrine-Disruptor, Pharmaceutical, and Personal Care Product Chemicals during Simulated Drinking Water Treatment Processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Frimmel, F.H. Oxidative treatment of pharmaceuticals in water. Water Res. 2000, 34, 1881–1885. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, S.P.; Wang, G.L.; Qiao, L.P. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigments 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Sun, J.H.; Dong, S.Y.; Wang, Y.K.; Sun, S.P. Preparation and photocatalytic property of a novel dumb bell-shaped ZnO microcrystal photocatalyst. J. Hazard. Mater. 2009, 172, 1520–1526. [Google Scholar] [CrossRef]
- Ekemena, O.; Oseghe, A.; Ofomaja, E. Facile microwave synthesis of pine cone derived C-doped TiO2 for the photodegradation of tetracycline hydrochloride under visible- LED light. J. Environ. Manag. 2018, 223, 860–867. [Google Scholar]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Periakaruppan, P.; Vellaichamy, B.; Nagulan, B. Efficacious separation of electron-hole pairs in CeO2–Al2O3 nanoparticles embedded GO heterojunction for robust visible-light driven dye degradation. J. Coll. Interf. Sci. 2018, 512, 219–230. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H.R. Study of photocatalytic activity of ZnS quantum dots as efficient nanoparticles for effect of ferric ion doping. Spectrochim. Acta Part A 2014, 22, 260–267. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H.R.; Khani, O. Pure and Fe3+-doped ZnS quantum dots as novel and efficient nanophotocatalysts: Synthesis, characterization and use for decolorization of Victoria blue R. Mater. Sci. Semicond. Process. 2013, 16, 1154–1161. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Farsi, M. Effect of transition metal ion doping on the photocatalytic activity of ZnS quantum dots: Synthesis, characterization, and application for dye decolorization. J. Mol. Catal. A Chem. 2015, 399, 53–61. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Farsi, M. Quantum dot based photocatalytic decolorization as an efficient and green strategy for the removal of anionic dye. Mater. Sci. Semicond. Process. 2015, 31, 478–486. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Karimia, F.; Kazemdehdashti, H.; Kavoshi, L. Fast Sonochemically-Assisted Synthesis of Pure and Doped Zinc Sulfide Quantum Dots and their Applicability in Organic Dye Removal from Aqueous Media. J. Photochem. Photobiol. 2018, 181, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Tripathi, V.K.; Singh, K.K.; Bhatia, T.; Upadhyay, N.K.; Goyal, B.; Pandey, G.; Hwang, I.; Tandon, P. Nitrogen donor ligand for capping ZnS quantum dots: A quantum chemical and toxicological insight. RSC Adv. 2019, 9, 28510–28524. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Wu, H.; He, X.; Chen, L.; Zhang, Y. A Highly Sensitive Fluorescent Turn-On Biosensor for Glycoproteins Based on Boronic Acid Functional Polymer Capped Mn-Doped ZnS Quantum Dots. Anal. Chim. Acta 2017, 995, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Sani, A.R.; Bandegharaei, A.H.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Patel, J.; Jain, B.; Singh, A.K.; Susan, M.A.B.H.; Paul, L.J. Mn-Doped ZnS Quantum dots–An Effective Nanoscale Sensor. Microchem. J. 2020, 155, 104755. [Google Scholar] [CrossRef]
- Abha, K.; Nebu, J.; Anjali Devi, J.S.; Aparna, R.S.; Anjana, R.R.; Aswathy, A.O.; George, S. Photoluminescence Sensing of Bilirubin in Human Serum Using L-Cysteine Tailored Manganese Doped Zinc Sulphide Quantum Dots. Sens. Actuat.-B-Chem. 2019, 282, 300–308. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Ren, Y.; Fan, J. Cu-Mn Codoped ZnS Quantum Dots Based Ratiometric Fluorescent Sensor for Folic Acid. Anal. Chim. Acta 2018, 1040, 136–142. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhang, W.; Shao, Y.; Chen, T.; Sun, M.; Fu, X. Photocatalytic degradation of dyes by ZnIn2S4 microspheres under visible light irradiation. J. Phys. Chem. C 2009, 113, 4433–4440. [Google Scholar] [CrossRef]
- Wang, X.; Shen, S.; Jin, S.; Yang, J.; Li, M.; Wang, X.; Hana, H.; Li, C. Effects of Zn2+and Pb2+ dopants on the activity of Ga2O3-based photocatalysts for watersplitting. Phys. Chem. Chem. Phys. 2013, 15, 19380–19386. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.J.; Efros, A.L.; Erwin, S.C. Doped nanocrystals. Science 2008, 319, 1776–1779. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.N.; Gallagher, D.; Hong, X.; Nurmikko, A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 1994, 72, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yan, X.P. Doped quantum dots for chemo/biosensing and bioimaging. Chem. Soc. Rev. 2013, 42, 5489–5521. [Google Scholar] [CrossRef] [PubMed]
- Eilers, J.; Groeneveld, E.; de Mello Donega, C.; Meijerink, A. Optical Properties of Mn-Doped ZnTe magic size nanocrystals. J. Phys. Chem. Lett. 2012, 3, 1663–1667. [Google Scholar] [CrossRef]
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping semiconductor nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, O.; Angerhofer, A.; Cao, Y.C. Radial-Position-Controlled Doping in CdS/ZnS Core/Shell Nanocrystals. J. Am. Chem. Soc. 2006, 128, 12428–12429. [Google Scholar] [CrossRef]
- Pradhan, N.; Goorskey, D.; Thessing, J.; Peng, X.G. An alternative of CdSe nanocrystal emitters: Pure and tunable impurity emissions in ZnSenanocrystals. J. Am. Chem. Soc. 2005, 127, 17586–17587. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Tang, J.; Tang, W. Surface ligands engineering of semiconductor quantum dots for chemosensory and biological applications. Mater. Today 2017, 20, 360–376. [Google Scholar] [CrossRef]
- Zhou, C.; Song, J.; Zhou, L.; Zhong, L.; Liu, J.; Qi, Y. Greener Synthesis and Optimization of Highly Photoluminescence Mn2+-Doped ZnS Quantum Dots. J. Lumin. 2015, 158, 176–180. [Google Scholar] [CrossRef]
- Singhal, M.; Sharma, J.K.; Jeon, H.C.; Kang, T.W.; Kumar, S. Effect of Pyridine Capping on Morphological and Optical Properties of Zns:Mn2+ Core–Shell Quantum Dots. J. Mater. Sci. Mater. Electron. 2016, 27, 3003–3010. [Google Scholar] [CrossRef]
- La Porta, F.A.; Ferrer, M.M.; Santana, Y.V.B.; Raubach, C.W.; Longo, V.M.; Sambrano, J.R.; Longo, E.; Andres, J.; Li, M.S.; Varela, J.A. Synthesis of Wurtzite ZnS Nanoparticles Using the Microwave Assisted Solvothermal Method. J. Alloys Compd. 2013, 556, 153–159. [Google Scholar] [CrossRef]
- Qadri, S.B.; Skelton, E.F.; Hsu, D.; Dinsmore, A.D.; Yang, J.; Gray, H.F.; Ratna, B.R. Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 1999, 60, 9191–9193. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Tajarrod, N.; Farahani, M.M. A New Fluorescence Sensor for Cerium (III) Ion Using Glycine Dithiocarbamate Capped Manganese Doped ZnS Quantum Dots. J. Fluoresc. 2015, 25, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Piotrowska, H.; Murias, M.; Balan, L.; Moritz, M.; Lulek, J.; Schneider, R.J. Thioglycerol-capped Mn-doped ZnS quantum dot bioconjugates as efficient two-photon fluorescent nano-probes for bioimaging. Mater. Chem. B 2013, 1, 698–706. [Google Scholar] [CrossRef]
- Kolmykov, O.; Coulon, J.; Lalevée, J.; Alem, H.; Medjahdi, G.; Schneider, R. Aqueous Synthesis of Highly luminescent Glutathione-capped Mn2+ -doped ZnS Quantum Dots. Mater. Sci. Eng. C 2014, 44, 17–23. [Google Scholar] [CrossRef]
- Karikalan, V.; Panneerselvam, A.; Vallalperuman, K. Physico—Chemical Analysis on Cetylpyridinium Chloride (Cpc) with Alcohol Solution at Different Temperatures—Ultrasonic, UV and FTIR Analysis. Dig. J. Nanomater. Bios. 2018, 13, 115–128. [Google Scholar]
- Kung, K.H.S.; Hayes, K.F. Fourier Transform Infrared Spectroscopic Study of the Adsorption of Cetyltrimethylammonium Bromide and Cetylpyridinium Chloride on Silica. Langmuir 1993, 9, 263–267. [Google Scholar] [CrossRef]
- Sotelo-Gonzalez, E.; Roces, L.; Garcia-Granda, S.; Fernandez-Arguelles, M.T.; Costa-Fernandez, J.M.; Sanz-Medel, A. Influence of the Mn2+ concentration on Mn2+ -doped ZnS Quantum Dots Synthesis: Evaluation of the Structural and Photoluminescent Properties. Nanoscale 2013, 5, 9156–9161. [Google Scholar] [CrossRef]
- Shah, S.I.; Li, W.; Huang, C.P.; Jung, O.; Ni, C. Study of Nd3+, Pd2+, Pt4+, and Fe3+Dopant Effect on Photoreactivity of TiO2 Nanoparticle. PNAS 2002, 99, 6482–6486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauc, J. Optical Properties of Solids; Academic Press Inc.: New York, NY, USA, 1966. [Google Scholar]
- Pouretedal, H.R.; Keshavarz, M.H.; Yosefi, M.H.; Shokrollahi, A.; Zali, A. Photodegradation of HMX and RDX in the Presence of Nanocatalyst of Zinc Sulfide Doped with Copper. Iran. J. Chem. Chem. Eng. 2009, 28, 13–19. [Google Scholar]
- Pourahmad, A. Photocatalytic Activity of Quantum Dots Incorporated in Molecular Sieves for Generation of Hydrogen. Spectrochim. Acta A 2012, 94, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Veeravazhuthi, V.; Muthukumarasamy, N.; Thambidurai, M.; Vishnu Shankar, D. Effect of nickel doping on structural and optical properties of ZnS nanoparticles. Superlattice Microst. 2015, 86, 552–558. [Google Scholar] [CrossRef]
- Guo, C.; Gao, S.; Lv, J.; Hou, S.; Zhang, Y.; Xu, J. Assessing the photocatalytic transformation of norfloxacin by BiOBr/iron oxides hybrid photocatalyst: Kinetics, intermediates, and influencing factors. Appl. Catal. B-Environ. 2017, 205, 68–77. [Google Scholar] [CrossRef]
- Ahmad, I.; Bano, R.; Musharraf, S.G.; Sheraz, M.A.; Ahmed, S.; Tahir, H.; Arfeen, Q.; Bhatti, M.S.; Shad, Z.; Hussain, S.F. Photodegradation of Norfloxacin in aqueous and organic solvents: A kinetic study. J. Photochem. Photobiol. A 2015, 302, 1–10. [Google Scholar] [CrossRef]
- Park, H.R.; Chung, K.Y.; Lee, H.C.; Lee, J.K.; Bark, K.M. Ionization and Divalent Cation Complexation of Quinolone Antibiotics in Aqueous Solution. Bull. Korean Chem. Soc. 2000, 21, 849. [Google Scholar]
- D’Andrea, G.; Di Nicolantonio, G. A Graphical Approach to Determine the Isoelectric Point and Charge of Small Peptides from pH 0 to 14. J. Chem. Educ. 2002, 79, 972. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Coll. Interf. Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef]
- Lair, A.; Ferronato, C.; Chovelon, J.M.; Herrmann, J.M. Naphthalene degradation in water by heterogeneous photocatalysis: An investigation of the influence of inorganic anions. J. Photochem. Photobiol. A Chem. 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Wang, C.C.; Lee, C.K.; Lyu, M.D.; Juang, L.C. Photocatalytic degradation of C.I. Basic Violet 10 using TiO2 catalysts supported by Y zeolite: An investigation of the effects operational parameters. Dyes Pigments 2008, 76, 817–842. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Asilturka, M.; Sayılkana, F.; Arpac, E. Effect of Fe3+ ion doping to TiO2 on the photocatalytic degradation of Malachite Green dye under UV and vis-irradiation. J. Photochem. Photobiol. A 2009, 203, 64–71. [Google Scholar] [CrossRef]
- Mahyari, M.; Bide, Y.; Gavgani, J.N. Iron(III) porphyrin supported on S and N codoped graphene quantum dot as an efficient photocatalyst for aerobic oxidation of alcohols under visible light irradiation. Appl. Catal. A 2016, 157, 100–109. [Google Scholar]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and characterization of nitrogen doped TiO2 nanophotocatalyst with high visible light activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Khani, O.; Shamsipur, M.; Vatanpour, V. High-performance pure and Fe3+-ion doped ZnS quantum dots as green nanophotocatalysts for the removal of malachite green under UV-light irradiation. J. Hazard. Mater. 2013, 250–251, 370–378. [Google Scholar] [CrossRef]
- Beydoun, D.; Amal, R.; Low, G.; Evoy, S.M. Role of nanoparticles in photocatalysis. J. Nanopart. Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Sun, L.; Liu, C.; Liao, C.; Yan, C. ZnS nanoparticles doped with Cu(I) by controlling coordination and precipitation in aqueous solution. J. Mater. Chem. 1999, 9, 1655–1657. [Google Scholar] [CrossRef]
- Montazerozohori, M.; Nasr-Esfahani, M.; Joohari, S. Photocatalytic degradation of an organic dye in some aqueous buffer solutions using nano titanium dioxide: A kinetic study. Environ. Prot. Eng. 2012, 38, 45–55. [Google Scholar] [CrossRef]
- Kumar, K.; Chitkara, M.; Sandhua, I.S.; Mehta, D.; Kumar, S. Photocatalytic and magnetic properties of Zn1-xCrxO nanocomposites prepared by coprecipitation method. Mater. Sci. Semicond. Proc. 2015, 30, 142–151. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl. Catal. B Environ. 2004, 57, 23–30. [Google Scholar] [CrossRef]
- Kaur, A.; Kansal, S.K. Bi2WO6nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wong, K.H.; Zhang, D.Q.; Hu, C.; Yu, J.C.; Chan, C.Y.; Wong, P.K. Zn:In(OH)ySz solid solution nanoplates: Synthesis, characterization, and photocatalytic mechanism. Environ. Sci. Technol. 2009, 43, 7883–7888. [Google Scholar] [CrossRef]
- Guo, H.; Gao, N.; Yang, Y.; Zhang, Y. Kinetics and transformation pathways on oxidation of fluoroquinolones with thermally activated persulfate. Chem. Eng. J. 2016, 292, 82–91. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO2 heterogeneous catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef]
- Chen, M.; Chu, W. Photocatalytic degradation and decomposition mechanism of fluoroquinolones norfloxacin over bismuth tungstate: Experiment and mathematic model. Appl. Catal. B Environ. 2015, 168–169, 175–182. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, T.; Wu, X.; Mao, J. Distinguishing homogeneous-heterogeneous degradation of norfloxacin in a photochemical Fenton-like system (Fe3O4/UV/ oxalate) and the interfacial reaction mechanism. Water Res. 2017, 119, 47–56. [Google Scholar] [CrossRef]
- Ding, D.H.; Liu, C.; Ji, Y.F.; Yang, Q.; Chen, L.L.; Jiang, C.L.; Cai, T.M. Mechanism insight of degradation of norfloxacin by magnetite nanoparticles activated persulfate: Identification of radicals and degradation pathway. Chem. Eng. J. 2017, 308, 330–339. [Google Scholar] [CrossRef]
- Guo, H.G.; Ke, T.L.; Gao, N.Y.; Liu, Y.; Cheng, X. Enhanced degradation of aqueous norfloxacin and enrofloxacin by UV-activated persulfate: Kinetics, pathways and deactivation. Chem. Eng. J. 2017, 316, 471–480. [Google Scholar] [CrossRef]
- Liu, C.; Nanaboina, V.; Korshin, G.V.; Jiang, W. Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater. Water Res. 2012, 46, 5235–5246. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, R.P.; Jia, M.K.; Wang, S.L.; Huang, Y.P.; Chen, C.C. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway. Chem. Eng. J. 2015, 274, 290–297. [Google Scholar] [CrossRef]
- Gou, J.F.; Ma, Q.L.; Deng, X.Y.; Cui, Y.Q.; Zhang, H.X.; Cheng, X.W.; Li, X.L.; Xie, M.Z.; Cheng, Q.F. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017, 308, 818–826. [Google Scholar] [CrossRef]
- Tang, L.; Wang, J.J.; Zeng, G.M.; Liu, Y.N.; Deng, Y.C.; Zhou, Y.Y.; Tang, J.; Wang, J.J.; Guo, Z. Enhanced photocatalytic degradation of norfloxacin in aqueous Bi2WO6 dispersions containing nonionic surfactant under visible light irradiation. J. Hazard. Mater. 2016, 306, 295–304. [Google Scholar] [CrossRef]
- Yang, H.; Mei, L.; Wang, P.; Genereux, J.; Wang, Y.; Yi, B.; Au, C.; Dang, L.; Feng, P. Photocatalytic degradation of norfloxacin on different TiO2-X polymorphs under visible light in water. RSC Adv. 2017, 7, 45721–45732. [Google Scholar] [CrossRef] [Green Version]
- Shankaraiah, G.; Poodari, S.; Bhagawan, D.; Himabindu, V.; Vidyavathi, S. Degradation of antibiotic norfloxacin in aqueous solution using advanced oxidation processes (AOPs)—A comparative study. Desalin. Water Treat. 2016, 57, 27804–27815. [Google Scholar] [CrossRef]
- Nekouei, S.; Nekouei, F. Photocatalytic degradation of norfloxacin and its intermediate degradation products using nitrogen–doped activated carbon–CuS nanocomposite assisted by visible irradiation. Appl. Organometal. Chem. 2018, 32, e4418. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zou, L.; Liu, X. Efficient Degradation of Norfloxacin and Simultaneous Electricity Generation in a Persulfate-Photocatalytic Fuel Cell System. Catalysts 2019, 9, 835. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Xue, Y.; Wang, Q.; Wang, P.; Yao, H.; Zhang, W.; Zhao, J.; Li, Y. Photocatalytic oxidation of norfloxacin by Zn0.9Fe0.1S supported on Ni foam under visible light irradiation. Chemosphere 2019, 230, 406–415. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.M.; Khan, H.M.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ codoped ZnO with the addition of HSO5–: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, X.; Sun, P.; Lin, S.; Cao, W.; Li, Z.; Liu, W. Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity Evaluation. Chemosphere 2019, 237, 124433. [Google Scholar] [CrossRef]




















| Name | Molecular Structure | Mw (g/mol) | λmax | Molecular Formula |
|---|---|---|---|---|
| Norfloxacin |  | 319.33 | 275 nm | C16H18FN3O3 |
| Condition | Rate Constant k (min−1) | R2 | DE (%) |
|---|---|---|---|
| Blank | 5.0 × 10‒4 | 0.971 | 2.93 |
| Pure ZnS + Sunlight | 1.9 × 10‒2 | 0.989 | 66.73 |
| Mn:ZnS + Sunlight | 2.0 × 10‒2 | 0.9951 | 71.4 |
| Pure ZnS + UV | 2.77 × 10‒2 | 0.994 | 81.8 |
| Mn:ZnS + UV | 3.21 × 10‒2 | 0.989 | 86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.; Singh, A.K.; Carabineiro, S.A.C. Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors. Nanomaterials 2020, 10, 964. https://doi.org/10.3390/nano10050964
Patel J, Singh AK, Carabineiro SAC. Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors. Nanomaterials. 2020; 10(5):964. https://doi.org/10.3390/nano10050964
Chicago/Turabian StylePatel, Jyoti, Ajaya K. Singh, and Sónia. A. C. Carabineiro. 2020. "Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors" Nanomaterials 10, no. 5: 964. https://doi.org/10.3390/nano10050964
APA StylePatel, J., Singh, A. K., & Carabineiro, S. A. C. (2020). Assessing the Photocatalytic Degradation of Fluoroquinolone Norfloxacin by Mn:ZnS Quantum Dots: Kinetic Study, Degradation Pathway and Influencing Factors. Nanomaterials, 10(5), 964. https://doi.org/10.3390/nano10050964