Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Calcinated Ca2+/Al3+ Layered Double Hydroxide with Phosphonium-Based IL
2.3. Ring Opening Polymerization of ε-Caprolactone in the Presence of LDH
2.4. PCL-LDH Nanocomposites Preparation by Melt-Blending
2.5. Characterization Methods
3. Results and Discussion
3.1. Preparation of IL-Functionalized LDH Nanoparticles with Low-Water Content
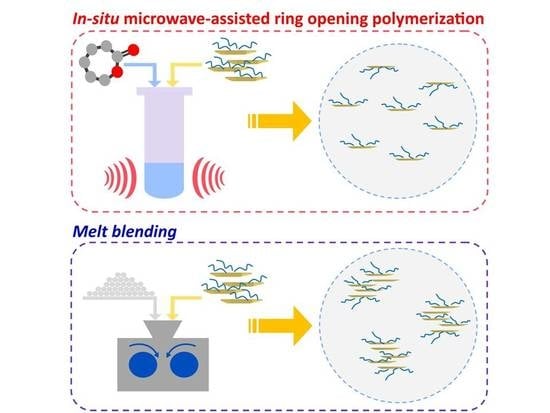
3.2. PCL-LDH Nanocomposites: In Situ ROP vs. Melt-Blending
3.3. Gas/vapor Properties of PCL-LDH Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demirkaya, Z.D.; Sengul, B.; Eroglu, M.S.; Dilsiz, N. Comprehensive characterization of polylactide-layered double hydroxides nanocomposites as packaging materials. J. Polym. Res. 2015, 22, 124. [Google Scholar] [CrossRef]
- Coiai, S.; Cicogna, F.; de Santi, A.; Perez Amaro, L.; Spiniello, R.; Signori, F.; Fiori, S.; Oberhauser, W.; Passaglia, E. MMT and LDH organo-modification with surfactants tailored for PLA nanocomposites. Express Polym. Lett. 2017, 11, 163–175. [Google Scholar] [CrossRef]
- Chakraborti, M.; Jackson, J.K.; Plackett, D.; Brunette, M.; Burt, H.M. Drug intercalation in layered double hydroxide clay: Application in the development of a nanocomposite film for guided tissue regeneration. Int. J. Pharm. 2011, 416, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Benes, H.; Kredatusova, J.; Peter, J.; Livi, S.; Bujok, S.; Pavlova, E.; Hodan, J.; Abbrent, S.; Konefał, M.; Ecorchard, P. Ionic Liquids as Delaminating Agents of Layered Double Hydroxide during In-Situ Synthesis of Poly (Butylene Adipate-co-Terephthalate) Nanocomposites. Nanomaterials 2019, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Kumar, S.; Kona, B.R.; van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Vieira, R.S.; Ferreira, S.O.; Reis, C. Preparation and evaluation of the Ca-Al layered double hydroxide for removal of copper(II), nickel(II), zinc(II), chromium(VI) and phosphate from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 5469–5480. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhai, X.; Xiong, P.; Kou, L.; Ji, X.; Lu, W. Prediction and synthesis of novel layered double hydroxide with desired basal spacing based on relevance vector machine. Mater. Res. Bull. 2017, 93, 123–129. [Google Scholar] [CrossRef]
- Li, S.; Ezugwu, C.I.; Zhang, S.; Xiong, Y.; Liu, S. Co-doped MgAl-LDHs nanosheets supported Au nanoparticles for complete catalytic oxidation of HCHO at room temperature. Appl. Surf. Sci. 2019, 487, 260–271. [Google Scholar] [CrossRef]
- Yan, S.; Wei, M. Polyfunctional Layered Materials, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 105–136. [Google Scholar]
- Laipan, M.; Yu, J.; Zhu, R.; Zhu, J.; Smith, A.T.; He, H.; O’Hare, D.; Sun, L. Functionalized layered double hydroxides for innovative applications. Mater. Horiz. 2020, 7, 715–745. [Google Scholar] [CrossRef]
- Barik, S.; Badamali, S.K.; Behera, L.; Jena, P.K. Mg–Al LDH reinforced PMMA nanocomposites: A potential material for packaging industry. Compos. Interfaces 2018, 25, 369–380. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bharadiya, P.; Hansora, D. Layered double hydroxide based bionanocomposites. Appl. Clay Sci. 2019, 177, 19–36. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Celli, A.; Aloisio, I.; Di Gioia, D.; Marek, A.A.; Verney, V.; Leroux, F. Dual chain extension effect and antibacterial properties of biomolecules interleaved within LDH dispersed into PBS by in situ polymerization. Dalton Trans. 2018, 47, 3155–3165. [Google Scholar] [CrossRef]
- Isaacs-Paez, E.D.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Martinez-Rosales, J.M.; Flores-Cano, J.B. Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions. Mechanism and effect of operating conditions. Chem. Eng. J. 2014, 245, 248–257. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, B.; Yu, J.; Xu, Z. Effect of calcination on adsorption performance of Mg–Al layered double hydroxide prepared by a water-in-oil microemulsion method. RSC Adv. 2016, 6, 50128–50137. [Google Scholar] [CrossRef]
- Pandey, J.K.; Reddy, K.R.; Mohanty, A.K.; Misra, M. Handbook of Polymer Nanocomposites: Processing, Performance and Application, Volume A: Layered Silicates, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; pp. 21–52. [Google Scholar]
- Kredatusova, J.; Benes, H.; Livi, S.; Pop-Georgievski, O.; Ecorchard, P.; Abbrent, S.; Pavlova, E.; Bogdał, D. Influence of ionic liquid-modified LDH on microwave-assisted polymerization of ε-caprolactone. Polymer 2016, 100, 68–94. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Bujok, S.; Konefał, M.; Abbrent, S.; Pavlova, E.; Svoboda, J.; Trhlíková, O.; Walterová, Z.; Beneš, H. Ionic liquid-functionalized LDH as catalytic-initiating nanoparticles for microwave-activated ring opening polymerization of ε-caprolactone. React. Chem. Eng. 2020, 5, 506–518. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, H.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Review of time lag permeation technique as a method for characterization of porous media and membranes. Adsorption 1997, 3, 283–312. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinariab, M. Effect of organically modified Ni–Al layered double hydroxide loading on the thermal and morphological properties of l-methionine containing poly(amide-imide) nanocomposites. RSC Adv. 2015, 5, 28007–28013. [Google Scholar] [CrossRef]
- Narayanappa, A.N.; Kamath, P.V. Interaction of Pristine Hydrocalumite-Like Layered Double Hydroxides with Carbon Dioxide. ACS Omega 2019, 4, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, J.-A.; Choi, S.-J.; Oh, J.-M. Polymer Coated CaAl-Layered Double Hydroxide Nanomaterials for Potential Calcium Supplement. Int. J. Mol. Sci. 2014, 15, 22563–22579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manohara, G.V.; Maroto-Valer, M.M.; Garcia, S. The effect of the layer-interlayer chemistry of LDHs on developing high temperature carbon capture materials. Dalton Trans. 2020, 49, 923–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic Liquid as Surfactant Agent of Hydrotalcite: Influence on the Final Properties of Polycaprolactone Matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahiaoui, F.; Benhacine, F.; Ferfera-Harrar, H.; Habi, A.; Hadj-Hamou, A.S.; Grohens, Y. Development of antimicrobial PCL/nanoclay nanocomposite films with enhanced mechanical and water vapor barrier properties for packaging applications. Polym. Bull. 2015, 72, 235–254. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Nucleating Agents, 1st ed.; ChemTec Publishing: Scarborough, ON, Canada, 2016; pp. 33–52. [Google Scholar]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties: Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta 2007, 453, 75–96. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.-A.; Alexandre, M.; Degee, P.; Henrist, C.; Rulmont, A.; Dubois, P. New nanocomposite materials based on plasticized poly(L-lactide) and organo-modified montmorillonites: Thermal and morphological study. Polymer 2003, 44, 443–450. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Lopes-da-Silva, J.A.; Freire, M.G.; Coutinho, J.A.P. Surface tension and refractive index of pure and water-saturated tetradecyltrihexylphosphonium-based ionic liquids. J. Chem. Thermodyn. 2013, 57, 372–379. [Google Scholar] [CrossRef]
- Boesel, L.F.; LeMeur, S.; Thöny-Meyer, L.; Ren, Q. The effect of molecular weight on the material properties of biosynthesized poly(4-hydroxybutyrate). Int. J. Biol. Macromol. 2014, 71, 124–130. [Google Scholar] [CrossRef]
- Pepels, M.P.F.; Govaert, L.E.; Duchateau, R. Influence of the Main-Chain Configuration on the Mechanical Properties of Linear Aliphatic Polyesters. Macromolecules 2015, 48, 5845–5854. [Google Scholar] [CrossRef]
- Poláková, L.; Sedláková, Z.; Ecorchard, P.; Pavlova, E.; Peter, J.; Paruzel, B.; Beneš, H. Poly(meth)acrylate nanocomposite membranes containing in situ exfoliated graphene platelets: Synthesis, characterization and gas barrier properties. Eur. Polym. J. 2017, 94, 431–445. [Google Scholar] [CrossRef]
- Giel, V.; Galajdová, B.; Popelková, S.; Kredatusová, J.; Trchová, M.; Pavlova, E.; Beneš, H.; Válek, R.; Peter, J. Gas transport properties of novel mixed matrix membranes made of titanate nanotubes and PBI or PPO. Desalin. Water Treat. 2015, 56, 3285–3293. [Google Scholar] [CrossRef]
- Luduena, L.N.; Vazquez, A.; Alvarez, V.A. Effect of the Type of Clay Organo-Modifier on the Morphology, Thermal/Mechanical/Impact/Barrier Properties and Biodegradation in Soil of Polycaprolactone/Clay Nanocomposites. J. Appl. Polym. Sci. 2013, 128, 2648–2657. [Google Scholar] [CrossRef]
- He, H.; Kang, H.; Ma, S.; Bai, Y.; Yang, X. High adsorption selectivity of ZnAl layered double hydroxides and the calcined materials toward phosphate. J. Colloid Interf. Sci. 2010, 343, 225–231. [Google Scholar] [CrossRef] [PubMed]




| Sample ID | Sample Description |
|---|---|
| MB-PCL | Neat commercial PCL |
| MB-PCL + C-Ca/Al | Commercial PCL containing 1.0 wt % of C-Ca/Al LDH prepared by melt blending technique |
| MB-PCL + C-Ca/Al-D | Commercial PCL containing 1.0 wt % of C-Ca/Al-D LDH prepared by melt blending technique |
| ROP-PCL | PCL prepared by in situ ROP under microwave irradiation |
| ROP-PCL + C-Ca/Al | PCL prepared by in situ ROP under microwave irradiation in the presence of1.0 wt % of C-Ca/Al LDH |
| ROP-PCL + C-Ca/Al-D | PCL prepared by in situ ROP under microwave irradiation in the presence of 1.0 wt % of C-Ca/Al-D LDH |
| Sample | D(110) [Å] | Tα [°C] | Tm [°C] | ΔHm [J/g] | Xc [%] | Td5% [°C] | θH2O [o] |
|---|---|---|---|---|---|---|---|
| MB-PCL | 73 | −54.1 | 55.9 | 54.5 | 42.3 | 354.0 | 90 ± 3 |
| MB-PCL + C-Ca/Al | 161 | −54.4 | 53.6 | 59.6 | 42.8 | 307.1 | 81 ± 4 |
| MB-PCL + C-Ca/Al-D | 179 | −55.6 | 55.9 | 64.3 | 46.2 | 318.1 | 82 ± 3 |
| ROP-PCL | 86 | −57.5 | 53.6 | 65.1 | 46.8 | 304.3 | 81 ± 1 |
| ROP-PCL + C-Ca/Al | 188 | −56.8 | 55.4 | 51.2 | 37.2 | 308.0 | 75 ± 3 |
| ROP-PCL + C-Ca/Al-D | 245 | −55.4 | 56.9 | 59.0 | 42.9 | 318.7 | 79 ± 2 |
| Sample | Mn∙103 [g/mol] | Ð | Young Modulus, E [MPa] | Tensile Strength, σmax [MPa] | Elongation at Break, εmax [%] |
|---|---|---|---|---|---|
| MB-PCL | 126 | 1.22 | 443.0 ± 9.1 | 32.6 ± 2.5 | 424 ± 22.4 |
| MB-PCL + C-Ca/Al | 117 | 1.28 | 434.0 ± 7.6 | 28.7 ± 5.2 | 382 ± 59.2 |
| MB-PCL + C-Ca/Al-D | 120 | 1.31 | 465.0 ± 10.4 | 31.7 ± 1.8 | 419 ± 18.5 |
| ROP-PCL | 99 | 1.43 | 463.0 ± 12.6 | 28.2 ± 1.4 | 390 ± 12.7 |
| ROP-PCL + C-Ca/Al | 79 | 1.42 | 560.0 ± 7.1 | 17.7 ± 13.2 | 353 ± 77.0 |
| ROP-PCL + C-Ca/Al-D | 61 | 1.59 | 572.0 ± 26.2 | 25.4 ± 2.0 | 423 ± 23.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujok, S.; Hodan, J.; Beneš, H. Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques. Nanomaterials 2020, 10, 969. https://doi.org/10.3390/nano10050969
Bujok S, Hodan J, Beneš H. Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques. Nanomaterials. 2020; 10(5):969. https://doi.org/10.3390/nano10050969
Chicago/Turabian StyleBujok, Sonia, Jiří Hodan, and Hynek Beneš. 2020. "Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques" Nanomaterials 10, no. 5: 969. https://doi.org/10.3390/nano10050969
APA StyleBujok, S., Hodan, J., & Beneš, H. (2020). Effects of Immobilized Ionic Liquid on Properties of Biodegradable Polycaprolactone/LDH Nanocomposites Prepared by In Situ Polymerization and Melt-Blending Techniques. Nanomaterials, 10(5), 969. https://doi.org/10.3390/nano10050969