Detection of Human p53 In-Vitro Expressed in a Transcription-Translation Cell-Free System by a Novel Conjugate Based on Cadmium Sulphide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Synthesis of CdS-BSA QDs in Aqueous Solution
2.4. Conjugation QD-BSA-α-GST
2.5. Clones and Plasmids
2.6. Human In Vitro p53 Recombinant Protein IVTT Production
2.7. Enrichment of Human GST-Tagged Recombinant Protein Expressed by the IVTT System
2.8. SDS-PAGE and Immunoblotting
3. Results and Discussion
3.1. Physical and Chemical Characterizations of QDs
3.1.1. Absorption and Fluorescence Spectra
3.1.2. Effect of the pH in the Medium on the Synthesis of CdS-BSA QDs.
3.1.3. Effect of the pH of the Medium on the Synthesis of CdS QDs at 4 °C
Influence of Temperature
Influence of the Molar Cd2+/thioacetamide Ratio at 4 °C
Influence of the Molar Cd2+/BSA Ratio at 4 °C
3.2. Characterization of the CdS QDs
3.3. Conjugation QD-BSA-α-GST
3.4. Human Recombinant Protein IVTT Expressed
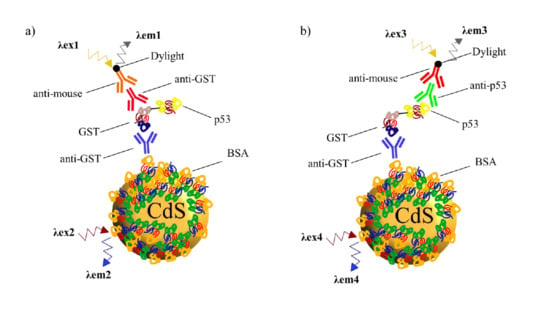
3.5. Characterization of the QD-GST-p53 Conjugated
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle—Biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid Interface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Canosa, J.B.; Wu, M.; Susumu, K.; Petryayeva, E.; Jennings, T.L.; Dawson, P.E.; Algar, W.R.; Medintz, I.L. Recent progress in the bioconjugation of quantum dots. Coord. Chem. Rev. 2014, 263, 101–137. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Lan, X.; Li, S.; Wu, D.; Han, T.; Zhong, H.; Zhang, Z. Synthesis of high quality and stability CdS quantum dots with overlapped nucleation-growth process in large scale. J. Colloid Interface Sci. 2011, 354, 15–22. [Google Scholar] [CrossRef]
- Barglik-Chory, C.; Buchold, D.; Schmitt, M.; Kiefer, W.; Heske, C.; Kumpf, C.; Fuchs, O.; Weinhardt, L.; Stahl, A.; Umbach, E. Synthesis, structure and spectroscopic characterization of water-soluble CdS nanoparticles. Chem. Phys. Lett. 2003, 379, 443–451. [Google Scholar] [CrossRef]
- Khomane, R.B.; Manna, A.; Mandale, A.B.; Kulkarni, B.D. Synthesis and characterization of dodecanethiol-capped cadmium sulfide nanoparticles in a Winsor II microemulsion of diethyl ether/AOT/water. Langmuir 2002, 18, 8237–8240. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; González, J.C.; Mansur, A.A. Biomolecule-quantum dot systems for bioconjugation applications. Colloids Surf. B 2011, 84, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, R.; Zhao, X.; Yang, B.; Gao, C.; Hao, X.; Wu, Y. New strategy for the evaluation of CdTe quantum dot toxicity targeted to bovine serum albumin. Sci. Total Environ. 2009, 407, 5019–5023. [Google Scholar] [CrossRef]
- He, X.; Gao, L.; Ma, N. One-step instant synthesis of protein-conjugated quantum dots at room temperature. Sci. Rep. 2013, 3, 2825. [Google Scholar] [CrossRef] [Green Version]
- Medintz, I.L.; Clapp, A.R.; Brunel, F.M.; Tiefenbrunn, T.; Uyeda, H.T.; Chang, E.L.; Deschamps, J.R.; Dawson, P.E.; Mattoussi, H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot–peptide conjugates. Nat. Mater. 2006, 5, 581–589. [Google Scholar] [CrossRef]
- Ma, N.; Sargent, E.H.; Kelley, S.O. Biotemplated nanostructures: Directed assembly of electronic and optical materials using nanoscale complementarity. J. Mater. Chem. 2008, 18, 954–964. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Silva, C.; Imasato, H.; Tabak, M. Interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants: Spectroscopy and modelling. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 2002, 1594, 84–99. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, S.; Ma, J.; Su, W.; Li, P.; Cui, J.; Liu, Y. Systematically investigation of interactions between BSA and different charge-capped CdSe/ZnS quantum dots. J. Photochem. Photobiol. A 2012, 249, 53–60. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Harvey, I.; Bunyan, K.E.; Stewart, A.J.; Sleep, D.; Harrison, D.J.; Berezenko, S.; Sadler, P.J. Structure, properties, and engineering of the major zinc binding site on human albumin. J. Biol. Chem. 2009, 284, 23116–23124. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Shen, Q.; Zhou, J.; Jiang, K. Biomimetic synthesis of CdS nanocrystals in the pepsin solution. Mater. Lett. 2005, 59, 2889–2892. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, M.; Guleria, A.; Singh, A.K.; Adhikari, S.; Rath, M.C. An insight into the optical properties of CdSe quantum dots during their growth in bovine serum albumin solution. J. Lumin. 2016, 179, 122–131. [Google Scholar] [CrossRef]
- Mamedova, N.N.; Kotov, N.A.; Rogach, A.L.; Studer, J. Albumin—CdTe nanoparticle bioconjugates: Preparation, structure, and interunit energy transfer with antenna effect. Nano Lett. 2001, 1, 281–286. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, F.; Fang, T.; Niu, W.; Liu, P.; Min, X.; Li, X. Bovine serum albumin-directed synthesis of biocompatible CdSe quantum dots and bacteria labeling. J. Colloid Interface Sci. 2011, 355, 9–14. [Google Scholar] [CrossRef]
- Huang, D.; Geng, F.; Liu, Y.; Wang, X.; Jiao, J.; Yu, L. Biomimetic interactions of proteins with functionalized cadmium sulfide quantum dots. Colloids Surf. Phys. Eng. Asp. 2011, 392, 191–197. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Almendral Parra, M.J.; Manzano-Roman, R.; Fuentes, M. Interactions of nanoparticles and biosystems: Microenvironment of nanoparticles and biomolecules in nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvatovich, P.; Veégvaári, A.; Saul, J.; Park, J.G.; Qiu, J.; Syring, M.; Pirrotte, P.; Petritis, K.; Tegeler, T.J.; Aziz, M. In vitro transcription/translation system: A versatile tool in the search for missing proteins. J. Proteome Res. 2015, 14, 3441–3451. [Google Scholar] [CrossRef]
- Yu, K.; Zaman, B.; Taal, R.; Ripmeester, J.A. Colloidal CdSe nanocrystals from tri-n-octylphosphine with various Cd sources: Control of a slow growth for high-quality and large-scale production. J. Cryst. Growth 2005, 283, 115–123. [Google Scholar] [CrossRef]
- Almendral-Parra, M.J.; Alonso-Mateos, A.; Boyero-Benito, J.F.; Sánchez-Paradinas, S.; Rodríguez-Fernández, E. A Novel Approach to the Fabrication of CdSe Quantum Dots in Aqueous Solution: Procedures for Controlling Size, Fluorescence Intensity, and Stability over Time. J. Nanomater. 2014, 2014, 397469. [Google Scholar] [CrossRef]
- Ahmed, M.; Guleria, A.; Rath, M.C.; Singh, A.K.; Adhikari, S.; Sarkar, S.K. Facile and green synthesis of CdSe quantum dots in protein matrix: Tuning of morphology and optical properties. J. Nanosci. Nanotechnol. 2014, 14, 5730–5742. [Google Scholar] [CrossRef]
- Vasudevan, D.M.; Sreekumari, S.; Vaidyanathan, K. Textbook of Biochemistry for Medical Students, 9th ed.; Jaypee Brothers Medical Publishers Pvt Ltd.: New Delhi, India, 2019. [Google Scholar]
- Almendral-Parra, M.J.; Alonso-Mateos, A.; Sánchez-Paradinas, S.; Boyero-Benito, J.F.; Rodríguez-Fernández, E.; Criado-Talavera, J.J. Procedures for Controlling the Size, Structure and Optical Properties of CdS Quantum Dots during Synthesis in Aqueous Solution. J. Fluoresc. 2012, 22, 59–69. [Google Scholar] [CrossRef]
- Cao, Y.C.; Wang, J. One-Pot Synthesis of High-Quality Zinc-Blende CdS Nanocrystals. J. Am. Chem. Soc. 2004, 126, 14336–14337. [Google Scholar] [CrossRef] [PubMed]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. Acs Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, N.; Saion, E.; Hussein, M.Z.; Erfani, M.; Rezaee, K.; Bahmanrokh, G. Phase Controlled Monodispersed CdS Nanocrystals Synthesized in Polymer Solution Using Microwave Irradiation. J. Inorg. Organomet. Polym. 2012, 22, 830–836. [Google Scholar] [CrossRef]
- Palanisamy, B.; Paul, B.; Chang, C.H. The synthesis of cadmium sulfide nanoplatelets using a novel continuous flow sonochemical reactor. Ultrason. Sonochem. 2015, 26, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Bao, N.; Shen, L.; Takata, T.; Domen, K.; Gupta, A.; Yanagisawa, K.; Grimes, C.A. Facile Cd− thiourea complex thermolysis synthesis of phase-controlled CdS nanocrystals for photocatalytic hydrogen production under visible light. J. Phys. Chem. C 2007, 111, 17527–17534. [Google Scholar] [CrossRef]
- Costa, S.; Almeida, A.; Castro, A.; Domingues, L. Fusion tags for protein solubility, purification, and immunogenicity in Escherichia coli: The novel Fh8 system. Front. Microbiol. 2014, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Arevalo-Pinzon, G.; Gonzalez-Gonzalez, M.; Fernando Suarez, C.; Curtidor, H.; Carabias-Sanchez, J.; Muro, A.; LaBaer, J.; Alfonso Patarroyo, M.; Fuentes, M. Self-assembling functional programmable protein array for studying protein-protein interactions in malaria parasites. Malar. J. 2018, 17, 270. [Google Scholar] [CrossRef]
- Ki, M.; Pack, S.P. Fusion tags to enhance heterologous protein expression. Appl. Microbiol. Biotechnol. 2020, 104, 2411–2425. [Google Scholar] [CrossRef]
- Leon, I.E.; Diez, P.; Etcheverry, S.B.; Fuentes, M. Deciphering the effect of an oxovanadium(IV) complex with the flavonoid chrysin (VOChrys) on intracellular cell signalling pathways in an osteosarcoma cell line. Metallomics 2016, 8, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Diez, P.; Baran, E.J.; Etcheverry, S.B.; Fuentes, M. Decoding the anticancer activity of VO-clioquinol compound: The mechanism of action and cell death pathways in human osteosarcoma cells. Metallomics 2017, 9, 891–901. [Google Scholar] [CrossRef] [PubMed]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Vicente, V.; Parra, M.J.A.; Boyero-Benito, J.F.; Auría-Soro, C.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Furones-Cuadrado, Á.; Hernández, Á.-P.; Manzano-Román, R.; Fuentes, M. Detection of Human p53 In-Vitro Expressed in a Transcription-Translation Cell-Free System by a Novel Conjugate Based on Cadmium Sulphide Nanoparticles. Nanomaterials 2020, 10, 984. https://doi.org/10.3390/nano10050984
Barba-Vicente V, Parra MJA, Boyero-Benito JF, Auría-Soro C, Juanes-Velasco P, Landeira-Viñuela A, Furones-Cuadrado Á, Hernández Á-P, Manzano-Román R, Fuentes M. Detection of Human p53 In-Vitro Expressed in a Transcription-Translation Cell-Free System by a Novel Conjugate Based on Cadmium Sulphide Nanoparticles. Nanomaterials. 2020; 10(5):984. https://doi.org/10.3390/nano10050984
Chicago/Turabian StyleBarba-Vicente, Víctor, María Jesús Almendral Parra, Juan Francisco Boyero-Benito, Carlota Auría-Soro, Pablo Juanes-Velasco, Alicia Landeira-Viñuela, Álvaro Furones-Cuadrado, Ángela-Patricia Hernández, Raúl Manzano-Román, and Manuel Fuentes. 2020. "Detection of Human p53 In-Vitro Expressed in a Transcription-Translation Cell-Free System by a Novel Conjugate Based on Cadmium Sulphide Nanoparticles" Nanomaterials 10, no. 5: 984. https://doi.org/10.3390/nano10050984
APA StyleBarba-Vicente, V., Parra, M. J. A., Boyero-Benito, J. F., Auría-Soro, C., Juanes-Velasco, P., Landeira-Viñuela, A., Furones-Cuadrado, Á., Hernández, Á. -P., Manzano-Román, R., & Fuentes, M. (2020). Detection of Human p53 In-Vitro Expressed in a Transcription-Translation Cell-Free System by a Novel Conjugate Based on Cadmium Sulphide Nanoparticles. Nanomaterials, 10(5), 984. https://doi.org/10.3390/nano10050984