Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization Techniques
2.2. Chemicals and Reagents
2.3. Synthesis and Functionalization of Silica Mesoporous Nanoparticles (MSNs)
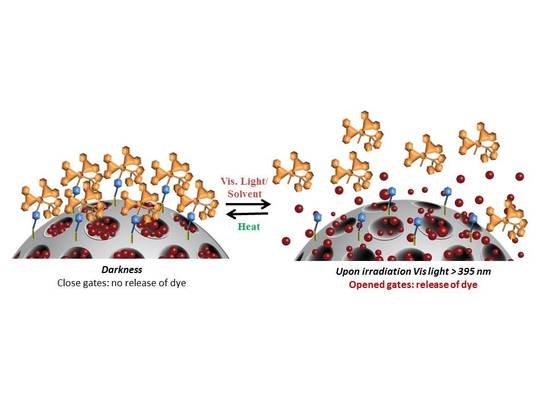
2.4. Cargo Release Controlled under Visible Light Irradiation and Stability Studies
3. Results and Discussion
3.1. Preparation of the Ruthenium(II) Gated-Mesoporous Silica Nanomaterials and Characterization
3.2. Visible Light-Controlled Release by Close–Open Gate Nanomaterials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Delivery Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Sancenón, F.; Pascual i Vidal, L.; Oroval Cucarella, M.; Aznar Gimeno, E.; Martínez-Máñez, R. Gated silica mesoporous materials in sensing applications. ChemistryOpen 2015, 4, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated materials for on-command release of guest molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous silica nanoparticles for drug delivery: Current insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneidinger, M.; Iturmendi, A.; Ulbricht, C.; Truglas, T.; Groiss, H.; Teasdale, I.; Salinas, Y. Mesoporous silica micromotors with a reversible temperature regulated on–off polyphosphazene switch. Macromol. Rapid Commun. 2019, 40, 1900328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Rama Rao, G.V.; Ward, T.L.; Lu, Y.; Lopez, G.P. Thermoresponsive transport through ordered mesoporous silica/PNIPAAm copolymer membranes and microspheres. Langmuir 2007, 23, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Mas, N.; Galiana, I.; Hurtado, S.; Mondragón, L.; Bernardos, A.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Abril-Utrillas, N.; Martínez-Máñez, R.; et al. Enhanced antifungal efficacy of tebuconazole using gated pH-driven mesoporous nanoparticles. Int. J. Nanomed. 2014, 9, 2597–2606. [Google Scholar]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Dong, L.; Peng, H.; Wang, S.; Zhang, Z.; Li, J.; Ai, F.; Zhao, Q.; Luo, M.; Xiong, H.; Chen, L. Thermally and magnetically dual-responsive mesoporous silica nanospheres: Preparation, characterization, and properties for the controlled release of sophoridine. J. Appl. Polym. Sci. 2014, 131, 40477–40484. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhu, D.; Zong, S.; Yang, L.; Zhong, Y.; Cui, Y. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer–Lipid bilayer. ACS Appl. Mater. Interfaces 2013, 5, 10895–10903. [Google Scholar] [CrossRef]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Amorós, P.; Guillem, C. pH- and photo-switched release of guest molecules from mesoporous silica supports. J. Am. Chem. Soc. 2009, 131, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Klan, P.; Solomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, S.; Chen, D.; Li, N.; Li, H.; Xu, Q.; Ge, J.; Lu, J. Light-triggered reversible assemblies of azobenzene-containing amphiphilic copolymer with β-cyclodextrin-modified hollow mesoporous silica nanoparticles for controlled drug release. Chem. Commun. 2012, 48, 10010–10012. [Google Scholar] [CrossRef]
- Zhang, Z.; Balogh, D.; Wang, F.; Tel-Vered, R.; Levy, N.; Sung, S.Y.; Nechushtai, R.; Willner, I. Light-induced and redox-triggered uptake and release of substrates to and from mesoporous SiO2 nanoparticles. J. Mater. Chem. B 2013, 1, 3159–3166. [Google Scholar] [CrossRef]
- Park, C.; Lee, K.; Kim, C. Photoresponsive cyclodextrin-covered nanocontainers and their sol-gel transition induced by molecular recognition. Angew. Chem. Int. Ed. 2009, 48, 1275–1278. [Google Scholar] [CrossRef]
- Offenloch, J.T.; Gernhardt, M.; Blinco, J.P.; Frisch, H.; Mutlu, H.; Barner-Kowollik, C. Contemporary photoligation chemistry: The visible light challenge. Chem. Eur. J. 2019, 25, 3700–3709. [Google Scholar] [CrossRef]
- San Miguel, V.; Álvarez, M.; Filevich, O.; Etchenique, R.; del Campo, A. Multiphoton reactive surfaces using ruthenium(II) photocleavable cages. Langmuir 2012, 28, 1217–1221. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Ji, Z.; Huang, Z.; Wu, Y. Molecular orbital engineering of a panchromatic cyclometalated Ru(II) dye for p-type dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 16518–16525. [Google Scholar] [CrossRef]
- Sun, W.; Thiramanas, R.; Slep, L.D.; Zeng, X.; Mailänder, V.; Wu, S. Photoactivation of anticancer Ru complexes in deep tissue: How deep can we go? Chem. Eur. J. 2017, 23, 10832–10837. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.Y. Functionalized mesoporous silica nanoparticle-based visible light responsive controlled release delivery system. Chem. Commun. 2011, 47, 2817–2819. [Google Scholar]
- Respondek, T.; Garner, R.N.; Herroon, M.K.; Podgorski, I.; Turro, C.; Kodanko, J.J. Light activation of a cysteine protease inhibitor: Caging of a peptidomimetic nitrile with RuII(bpy)2. J. Am. Chem. Soc. 2011, 133, 17164–17167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theis, S.; Iturmendi, A.; Gorsche, C.; Orthofer, M.; Lunzer, M.; Baudis, S.; Ovsianikov, A.; Liska, R.; Monkowius, U.; Teasdale, I. Metallo-supramolecular gels that are photocleavable with visible and near-infrared irradiation. Angew. Chem. Int. Ed. 2017, 56, 15857–15860. [Google Scholar] [CrossRef]
- Teasdale, I.; Theis, S.; Iturmendi, A.; Strobel, M.; Hild, S.; Jacak, J.; Mayrhofer, P.; Monkowius, U. Dynamic supramolecular ruthenium-based gels responsive to visible/NIR light and heat. Chem. Eur. J. 2019, 25, 9851–9855. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Sun, W.; Lu, H.; Kretzschmann, A.; Liu, J.; Wagner, M.; Butt, H.-J.; Deng, X.; Wu, S. Reconfiguring surface functions using visible-light-controlled metal-ligand coordination. Nat. Commun. 2018, 9, 3842. [Google Scholar] [CrossRef]
- Knežević, N.Ž. Visible light responsive anticancer treatment with an amsacrine-loaded mesoporous silica-based nanodevice. RSC Adv. 2013, 3, 19388–19392. [Google Scholar] [CrossRef]
- Frasconi, M.; Liu, Z.; Lei, J.; Wu, Y.; Strekalova, E.; Malin, D.; Ambrogio, M.W.; Chen, X.; Botros, Y.Y.; Cryns, V.L.; et al. Photoexpulsion of surface-grafted ruthenium complexes and subsequent release of cytotoxic cargos to cancer cells from mesoporous silica nanoparticles. J. Am. Chem. Soc. 2013, 135, 11603–11613. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, B.P.; Salmon, D.J.; Meyer, T.J. Mixed phosphine 2,2’-bipyridine complexes of ruthenium. Inorg. Chem. 1978, 17, 3334–3341. [Google Scholar] [CrossRef]
- Zayat, L.; Noval, M.G.; Campi, J.; Calero, C.I.; Calvo, D.J.; Etchenique, R. A new inorganic photolabile protecting group for highly efficient visible light GABA uncaging. ChemBioChem 2007, 8, 2035–2038. [Google Scholar] [CrossRef]
- Salinas, Y.; Hoerhager, C.; García-Fernández, A.; Resmini, M.; Sancenón, F.; Matínez-Máñez, R.; Brueggemann, O. Biocompatible phenylboronic-acid-capped ZnS nanocrystals designed as caps in mesoporous silica hybrid materials for on-demand pH-triggered release in cancer cells. ACS Appl. Mater. Interfaces 2018, 10, 34029–34038. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Candel, I.; Aznar, E.; Mondragón, L.; Torre, C.d.l.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Guillem, C.; Pérez-Payá, E.; et al. Amidase-responsive controlled release of antitumoral drug into intracellular media using gluconamide-capped mesoporous silica nanoparticles. Nanoscale 2012, 4, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Mondragón, L.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Barat, J.M.; Pérez-Payá, E.; Guillem, C.; et al. Enzyme-responsive intracellular controlled release using nanometric silica mesoporous supports capped with “saccharides”. ACS Nano 2010, 4, 6353–6368. [Google Scholar] [CrossRef] [PubMed]
- Nhavene, E.P.F.; Andrade, G.F.; Faria, J.A.Q.A.; Gomes, D.A.; Sousa, E.M.B.D. Biodegradable polymers grafted onto multifunctional mesoporous silica nanoparticles for gene delivery. ChemEngineering 2018, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-T.; Trewyn, B.G.; Wiench, J.W.; Pruski, M.; Lin, V.S.Y. Urea and thiourea-functionalized mesoporous silica nanoparticle catalysts with enhanced catalytic activity for Diels–Alder reaction. Top. Catal. 2010, 53, 187–191. [Google Scholar] [CrossRef]
- Yildirim, A.; Ozgur, E.; Bayindir, M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J. Mater. Chem. B 2013, 1, 1909–1920. [Google Scholar] [CrossRef] [Green Version]








| Sample | SBET m2/g | Pore vol.BJH cm3/g | Pore sizeBJH nm | HD[b] (PDI) nm | αdye mmol/gsilica | αlinker mmol/gsilica | αRC mmol/gsilica |
|---|---|---|---|---|---|---|---|
| MSN-0 | 836 | 0.67 | 3.96 | 126 ± 33 (0.069) | - | - | - |
| MSN1-NCO | 163 | 0.31 | - | 121 ± 28 (0.290) | 0.478 | 1.070 | - |
| MSN2-Py | 218 | 0.37 | - | 121 ± 30 (0.146) | 0.401 | 0.591 | - |
| MSN1-RC1 | 105 | 0.26 | - | 193 ± 61 (0.309) | 0.251 | 0.175 | 0.093 |
| MSN2-RC2 | 100 | 0.35 | - | 187 ± 107 (0.205) | 0.198 | 0.399 | 0.080 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, Y.; Brüggemann, O.; Monkowius, U.; Teasdale, I. Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 1030. https://doi.org/10.3390/nano10061030
Salinas Y, Brüggemann O, Monkowius U, Teasdale I. Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles. Nanomaterials. 2020; 10(6):1030. https://doi.org/10.3390/nano10061030
Chicago/Turabian StyleSalinas, Yolanda, Oliver Brüggemann, Uwe Monkowius, and Ian Teasdale. 2020. "Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles" Nanomaterials 10, no. 6: 1030. https://doi.org/10.3390/nano10061030
APA StyleSalinas, Y., Brüggemann, O., Monkowius, U., & Teasdale, I. (2020). Visible Light Photocleavable Ruthenium-Based Molecular Gates to Reversibly Control Release from Mesoporous Silica Nanoparticles. Nanomaterials, 10(6), 1030. https://doi.org/10.3390/nano10061030