Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide
Abstract
:1. Introduction
2. Experiment
2.1. The Catalysts Synthesis
2.2. The Catalysts Characterizations
2.3. Catalytic Activity Investigation
3. Results and Discussion
3.1. Compositional Analysis
3.2. Catalytic Performance toward CO Oxidation
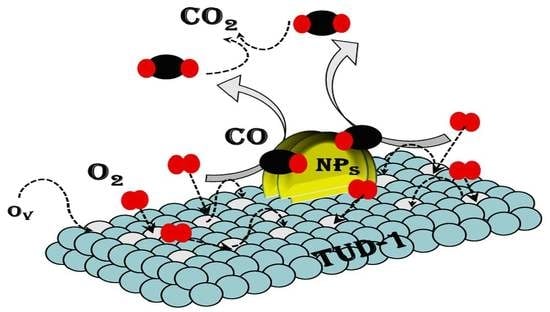
3.3. Proposed Mechanism
3.4. The Differences in Activity
3.5. Comparison with Other Supports
4. Conclusions and Viewpoint
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaneti, Y.V.; Tanaka, S.; Jikihara, Y.; Nakayama, T.; Bando, Y.; Haruta, M.; Yamauchi, Y. Room temperature carbon monoxide oxidation based on two-dimensional gold-loaded mesoporous iron oxide nanoflakes. Chem. Comm. 2018, 54, 8514–8517. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Song, L.; Wang, Y.; He, C.; Wang, Z.; Cui, L. High and stable catalytic activity of Ag/Fe2O3 catalysts derived from MOFs for CO oxidation. Mol. Catal. 2018, 447, 80–89. [Google Scholar] [CrossRef]
- Rijkeboer, R.C. Catalysts on cars− practical experience. Catal. Today 1991, 11, 141–150. [Google Scholar] [CrossRef]
- Lenaers, G. On-board real-life emission measurements on a 3-way catalyst gasoline car in motor way-, rural-and city traffic and on two Euro-1 diesel city buses. Sci. Total Environ. 1996, 189, 139–147. [Google Scholar] [CrossRef]
- Mizuno, N. (Ed.) Modern Heterogeneous Oxidation Catalysis: Design, Reactions and Characterization; John Wiley & Sons: Weinheim, Germany, 2009. [Google Scholar]
- Pillay, D.; Hwang, G.S. O2-coverage-dependent CO oxidation on reduced TiO2 (110): A first principles study. J. Chem. Phys. 2006, 125, 1447066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Flytzani-Stephanopoulos, M. Transition metal-promoted oxidation catalysis by fluorite oxides: A study of CO oxidation over Cu CeO2. Chem. Eng. J. Bio chem. Eng. J. 1996, 64, 283–294. [Google Scholar] [CrossRef]
- Eichler, A. CO oxidation on transition metal surfaces: Reaction rates from first principles. Surf. Sci. 2002, 498, 314–320. [Google Scholar] [CrossRef]
- Yao, Y.F.Y. Oxidation of alkanes over noble metal catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 293–298. [Google Scholar] [CrossRef]
- Oh, S.H.; Hoflund, G.B. Low-temperature catalytic carbon monoxide oxidation over hydrous and anhydrous palladium oxide powders. J. Catal. 2007, 245, 35–44. [Google Scholar] [CrossRef]
- Xanthopoulou, G.G.; Novikov, A.V.; Knysh, Y.A.; Amosov, A.P. Nanocatalysts for low-temperature oxidation of CO. Eurasian Chemico Technol. J. 2015, 17, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal-based catalysts: A review. Appl. Catal. B 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Srinivas, D.; Satyanarayana, C.V.V.; Manikandan, P.; Kumaran, R.S.; Sachin, M.; Shetti, V.N. Influence of the support on the preferential oxidation of CO in hydrogen-rich steam reformates over the CuO–CeO2–ZrO2 system. J. Catal. 2004, 221, 455–465. [Google Scholar] [CrossRef]
- Suchorski, Y.; Kozlov, S.M.; Bespalov, I.; Datler, M.; Vogel, D.; Budinska, Z.; Rupprechter, G. The role of metal/oxide interfaces for long-range metal particle activation during CO oxidation. Nat. Mater. 2018, 17, 519. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.M.; Gutiérrez, R.A.; Liu, Z.; Tenney, S.; Grinter, D.C.; Crumlin, E.; Senanayake, S.D.D. Invrse Catalysts for CO Oxidation: Enhanced Oxide–Metal Interactions in MgO/Au (111), CeO2/Au (111), and TiO2/Au (111). ACS Sustain. Chem. Eng. 2017, 5, 10783–10791. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, L.; Chen, M.; Lv, C.; Lian, X.; Wu, C.E.; Hu, X. CO Oxidation over Metal Oxide (La2O3, Fe2O3, PrO2, Sm2O3, and MnO2) Doped CuO-Based Catalysts Supported on Mesoporous Ce0.8Zr0.2O2 with Intensified Low-Temperature Activity. Catalysts 2019, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Kolobova, E.; Pestryakov, A.; Mamontov, G.; Kotolevich, Y.; Bogdanchikova, N.; Farias, M.; Corberan, V.C. Low-temperature CO oxidation on Ag/ZSM-5 catalysts: Influence of Si/Al ratio and redox pretreatments on formation of silver active sites. Fuel 2017, 188, 121–131. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Lv, T.; Wu, R.; Zhao, Y. Effect of precipitants on the catalytic performance of Pd–Cu/attapulgite clay catalyst for CO oxidation at room temperature and in humid circumstances. React. Kinet. Mech. Cat. 2018, 124, 203–216. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; el Rahman, S.K.A.; Ashour, S.S.; Hamdy, M.S. A review: The utilization of mesoporous materials in wastewater treatment. Mater. Res. Exp. 2019, 6, 122002. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Peng, H.; Xu, X.; Liu, W.; Wang, Z.; Wang, X. Ag supported on meso-structured SiO2 with different morphologies for CO oxidation: On the inherent factors influencing the activity of Ag catalysts. Microporous Mesoporous Mater. 2017, 242, 90–98. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Patel, A.; Rudolph, V.; Zhu, Z. Catalytic performance of Ru nanoparticles supported on different mesoporous silicas for preferential oxidation of CO in H2-rich atmosphere. Appl. Catal. A Gen. 2012, 447, 200–209. [Google Scholar] [CrossRef]
- Chilukuri, S.; Joseph, T.; Malwadkar, S.; Damle, C.Y.; Halligudi, S.B.; Rao, B.S.; Sastry, M.; Ratnasamy, P. Au and Au-Pt bimetallic nanoparticles in MCM-41 materials: Applications in co preferential oxidation. Stud. Surf. Sci. Catal. 2003, 146, 573–576. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Wang, Y.; Liu, N.; Zuo, Y.; Cui, L. Study of catalytic activity at the Ag/Al-SBA-15 catalysts for CO oxidation and selective CO oxidation. Chem. Eng. J. 2016, 283, 1097–1107. [Google Scholar] [CrossRef]
- Krishnan, C.K.; Nakamura, K.; Hirata, H.; Ogura, M. Pt/CeO2–ZrO2 present in the mesopores of SBA-15—A better catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2010, 12, 7513–7520. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; Altass, H.M.; Ashour, S.S.; Shkir, M.; el Rahman, S.K.A.; Hamdy, M.S. Enhancement the photocatalytic performance of semiconductors through composite formation with Eu-TUD-1. Optik 2020, 202, 163522. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. International union of pure commission on colloid and surface chemistry including catalysis reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Mubarak, A.T.; Alhanash, A.M.; Benaissa, M.; Hegazy, H.H.; Hamdy, M.S. In-situ activation of Pd-TUD-1 during the selective reduction of 1,5-cyclooctadiene. Microporous Mesoporous Mater. 2019, 278, 225–231. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; Khder, A.R.; Ashour, S.S.; Alhanash, A.M.; Shkir, M.; Hamdy, M.S. Effect of europium loading on the photoluminescence property of europium incorporated 3D-Mesoporous silica. J. Non Cryst. Solids 2019, 515, 68–74. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.N.; Nadagouda, M.N.; Varma, R.S. Photocatalytic CH activation and oxidative esterification using Pd@g-C3N4. Catal. Today 2018, 309, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.J.; Sun, T.Q.; Yang, L.P.; Sun, Y.G.; Bin, D.S.; Duan, S.Y.; Cao, A.M. A facile synthetic strategy for the creation of hollow noble metal/transition metal oxide nanocomposites. Chem. Commun. 2019, 5, 1076–1079. [Google Scholar] [CrossRef]
- Yildiz, Y.; Okyay, T.O.; Sen, B.; Gezer, B.; Kuzu, S.; Savk, A.; Sen, F. Highly monodisperse Pt/Rh nanoparticles confined in the graphene oxide for highly efficient and reusable sorbents for methylene blue removal from aqueous solutions. ChemistrySelect 2017, 2, 697–701. [Google Scholar] [CrossRef]
- Kumari, M.M.; Jacob, J.; Philip, D. Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectro. 2015, 137, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Pusztai, P.; Óvári, L.; Oszkó, A.; Erdőhelyi, A.; Papp, C.; Kiss, J. Probing the interaction of Rh, Co and bimetallic Rh–Co nanoparticles with the CeO2 support: Catalytic materials for alternative energy generation. Phys. Chem. Chem. Phys. 2015, 17, 27154–27166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunthaner, F.J.; Grunthaner, P.J.; Vasquez, R.P.; Lewis, B.; Maserjian, J.; Madhukar, A. Local atomic and electronic structure of oxide/GaAs and SiO2/Si interfaces using high-resolution XPS. J. Vac. Sci. Technol. 1979, 16, 1443–1453. [Google Scholar] [CrossRef]
- López, G.P.; Castner, D.G.; Ratner, B.D. XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Su, P.; Chen, Y.; Liu, X.; Chuai, H.; Liu, H.; Zhu, B.; Huang, W. Preparation and Characterization of Rh/MgSNTs Catalyst for Hydroformylation of Vinyl Acetate: The Rh0 was obtained by calcination. Catalysts 2019, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Simagina, V.I.; Storozhenko, P.A.; Netskina, O.V.; Komova, O.V.; Odegova, G.V.; Larichev, Y.V.; Ishchenko, A.V.; Ozerova, A.M. Development of catalysts for hydrogen generation from hydride compounds. Catalysts Today 2008, 138, 253–259. [Google Scholar] [CrossRef]
- Brun, M.; Berthet, A.; Bertolini, J.C. XPS, AES and Auger parameter of Pd and PdO. J. Electron Spectrosc. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Arico, A.S.; Shukla, A.K.; Kim, H.; Park, S.; Min, M.; Antonucci, V. An XPS study on oxidation states of Pt and its alloys with Co and Cr and its relevance to electroreduction of oxygen. Appl. Surf. Sci. 2001, 172, 33–40. [Google Scholar] [CrossRef]
- Tunc, I.; Suzer, S.; Correa-Duarte, M.A.; Liz-Marzan, L.M. XPS characterization of Au (core)/SiO2 (shell) nanoparticles. J. Phys. Chem. B 2005, 109, 7597–7600. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.; Tavares, P.B.; Pereira, M.F.; Órfão, J.J.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Schryer, D.R.; Upchurch, B.T.; Sidney, B.D.; Brown, K.G.; Hoflund, G.B.; Herz, R.K. A proposed mechanism for Pt/SnO sub x-catalyzed CO oxidation. J. Catal. 1991, 130. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, D.; Guo, J.; Zhu, B.; Huang, W.; Zhang, S. Improved Catalytic Performance of Au/α-Fe2O3-Like-Worm Catalyst for Low Temperature CO Oxidation. Nanomaterials 2019, 9, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.P.; Zhao, H.; Gao, Z.M.; Yuan, Z.Y. High-surface-area activated red mud supported Co3O4 catalysts for efficient catalytic oxidation of CO. RSC Adv. 2016, 6, 94748–94755. [Google Scholar] [CrossRef]
- Altass, H.M.; Khder, A.E.R.S. Catalytic oxidation of carbon monoxide over of gold-supported iron oxide catalyst. Mater. Res. Innov. 2018, 22, 107–114. [Google Scholar] [CrossRef]
- Tanaka, S.; Lin, J.; Kaneti, Y.V.; Yusa, S.I.; Jikihara, Y.; Nakayama, T.; Yamauchi, Y. Gold nanoparticles supported on mesoporous iron oxide for enhanced CO oxidation reaction. Nanoscale 2018, 10, 4779–4785. [Google Scholar] [CrossRef]
- Gu, D.; Tseng, J.C.; Weidenthaler, C.; Bongard, H.J.; Spliethoff, B.; Schmidt, W.; Schu’th, F. Gold on different manganese oxides: Ultra-low-temperature CO oxidation over colloidal gold supported on bulk-MnO2 nanomaterials. J. Am. Chem. Soc. 2016, 138, 9572–9580. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Zhao, W.; Xu, X.; Jin, Q.; Qi, J.; Wang, D. Enhanced catalytic activity of Au-CeO2/Al2O3 monolith for low-temperature CO oxidation. Catal. Commun. 2019, 129, 105729. [Google Scholar] [CrossRef]
- Dong, F.; Guo, Y.; Zhang, D.; Zhu, B.; Huang, W.; Zhang, S. Gold Nanoparticles Supported on Urchin-Like CuO: Synthesis, Characterization, and Their Catalytic Performance for CO Oxidation. Nanomaterials 2020, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Sciré, S. High-Performing Au-Ag Bimetallic Catalysts Supported on Macro-Mesoporous CeO2 for Preferential Oxidation of CO in H2-Rich Gases. Catalysts 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; He, L.; Wang, Y.; Gao, X.Q.; Li, W.C. Promotion effects of nickel-doped Al2O3-nanosheet-supported Au catalysts for CO oxidation. Chin. J. Catal. 2020, 41, 350–356. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.S.; Zanella, R. Comparative study of transition metal (Mn, Fe or Co) catalysts supported on titania: Effect of Au nanoparticle’s addition towards CO oxidation and soot combustion reactions. Chem. Eng. J. 2019, 385, 123848. [Google Scholar] [CrossRef]
- Morad, M.; Karim, M.A.; Altass, H.M.; Khder, A.E.R.S. Microwave-Assisted Synthesis of Gold Nanoparticles Supported on Mn3O4 Catalyst for Low Temperature CO Oxidation. Environ. Technol. 2019, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Kalwei, M.; Schüth, F.; Chao, K.J. Gold nanoparticles in SBA-15 showing catalytic activity in CO oxidation. Appl. Catal. A Gen. 2003, 254, 289–296. [Google Scholar] [CrossRef]
- Wu, H.; Pantaleo, G.; Venezia, A.; Liotta, L. Mesoporous silica-based gold catalysts: Novel synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs). Catalysts 2013, 3, 774–793. [Google Scholar] [CrossRef] [Green Version]
- Pachamuthu, M.P.; Srinivasan, V.V.; Maheswari, R.; Shanthi, K.; Ramanathan, A. The impact of the copper source on the synthesis of meso-structured CuTUD-1: A promising catalyst for phenol hydroxylation. Catal. Sci. Technol. 2013, 12, 3335–3342. [Google Scholar] [CrossRef]
- Kumar, R.; Das, P.P.; Al-Fatesh, A.S.; Fakeeha, A.H.; Pandey, J.K.; Chowdhury, B. Highly active InOx/TUD-1 catalyst towards Baeyer–Villiger oxidation of cyclohexanone using molecular oxygen and benzaldehyde. Catal. Commun. 2016, 74, 80–84. [Google Scholar] [CrossRef]
- Manjunathan, P.; Vijaykumar, S.M.; Prakash, C.; Atul, B.K.; Shubhangi, B.U.; Raman, R.; Ganapati, V.S. Mesoporous tin oxide: An efficient catalyst with versatile applications in acid and oxidation catalysis. Catal. Today 2018, 309, 61–76. [Google Scholar] [CrossRef]
- Fujita, T.; Ishida, T.; Shibamoto, K.; Honma, T.; Ohashi, H.; Murayama, T.; Haruta, M. CO Oxidation over Au/ZnO: Unprecedented Change of the Reaction Mechanism at Low Temperature Caused by a Different O2 Activation Process. ACS Catal. 2019, 9, 8364–8372. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Hinojosa-Reyes, M.; Nava, N.; Zanella, R. Efficient CO (carbon monoxide) oxidation using gold catalysts supported on WO3/titanate protonated nanotubes. Mater. Res. Bull. 2019, 115, 247–256. [Google Scholar] [CrossRef]






| Sample | Si/M Ratio | Texture Properties | |||
|---|---|---|---|---|---|
| Synthesis Mixture | Final Product | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | |
| TUD-1 | ∞ | ∞ | 655.2 | 1.710 | 4.5 |
| Pt-TUD-1 | 100 | 101.41 | 710.6 | 1.165 | 4.6 |
| Rh-TUD-1 | 100 | 101.21 | 685.9 | 0.804 | 5.9 |
| Au-TUD-1 | 100 | 107.06 | 644.1 | 0.970 | 7.5 |
| Pd-TUD-1 | 100 | 103.62 | 622.3 | 1.291 | 6.8 |
| Peaks of XPS Spectrum | Binding Energy (eV) | |||
|---|---|---|---|---|
| Rh-TUD-1 | Pd-TUD-1 | Pt-TUD-1 | Au-TUD-1 | |
| Si 2p | 102.9 | 102.73 | 102.8 | 102.76 |
| O 1s | 531.9 | 531.93 | 531.97 | 530.68 |
| Rh 3d | 5/2 = 307.6 3/2 = 311.8 | - | - | - |
| Pd 3d | - | 5/2 = 335.7 3/2 = 340 | - | - |
| Pt 4f | - | - | 5/2 = 71.76 7/2 = 74.97 | - |
| Au 4f | - | - | - | 5/2 = 84.42 7/2 = 88.04 |
| Ref. | Au Support | Au Loading % | Au Nanosize (nm) | Temperature (K) |
|---|---|---|---|---|
| Current work | TUD-1 | 0.94 | 5–10 nm | 303 K |
| [45] | α-FeOOH | 0.92 | 30 nm | 507 K |
| [46] | mesoporous Fe2O3 | 7.9 | 3–10 nm | 523 K |
| [47] | MnO2 | 1.0 | 1–3 nm | 321 K |
| [48] | CeO2/Al2O3 | 1.6 | 2–3 nm | 322 K |
| [49] | CuO | 1.0 | 4–8 nm | 353 K |
| [50] | CeO2 | 1.0 | 5–10 nm | 393 K |
| [51] | Ni/Al2O3 | 1.0 | 2.4–3.5 nm | 293 K |
| [52] | FeOx/TiO2 | 1.0 | 5 nm | 373 K |
| [53] | Mn3O4 | 4.0 | 30–40 nm | 371 K |
| [54] | SBA-15 | 4.8 | 10–50 nm | 433 K |
| [55] | MCM-41 | 4 | 5.1–6.9 nm | 454 K |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shehri, B.M.; Shkir, M.; Khder, A.S.; Kaushik, A.; Hamdy, M.S. Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide. Nanomaterials 2020, 10, 1067. https://doi.org/10.3390/nano10061067
Al-Shehri BM, Shkir M, Khder AS, Kaushik A, Hamdy MS. Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide. Nanomaterials. 2020; 10(6):1067. https://doi.org/10.3390/nano10061067
Chicago/Turabian StyleAl-Shehri, Badria M., Mohd Shkir, A. S. Khder, Ajeet Kaushik, and Mohamed S. Hamdy. 2020. "Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide" Nanomaterials 10, no. 6: 1067. https://doi.org/10.3390/nano10061067
APA StyleAl-Shehri, B. M., Shkir, M., Khder, A. S., Kaushik, A., & Hamdy, M. S. (2020). Noble Metal Nanoparticles Incorporated Siliceous TUD-1 Mesoporous Nano-Catalyst for Low-Temperature Oxidation of Carbon Monoxide. Nanomaterials, 10(6), 1067. https://doi.org/10.3390/nano10061067