W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Synthesis of W2C/WS2 NFs
2.3. Characterization
2.4. Electrochemical Measurements
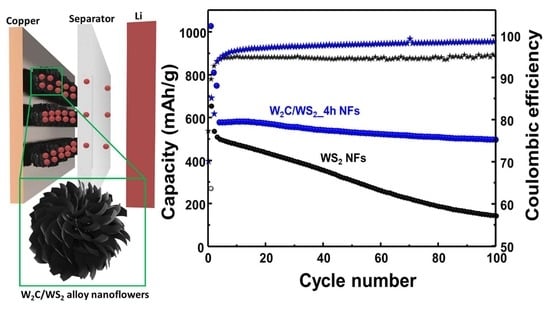
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Matte, H.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N. MoS2 and WS2 analogues of graphene. Angew. Chem. Int. Ed. Engl. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Two-Dimensional Transition-Metal. Dichalcogenides; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tuan Nguyen, D.M.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Le, Q.V. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Gatensby, R.; McEvoy, N.; Lee, K.; Hallam, T.; Berner, N.C.; Rezvani, E.; Winters, S.; O’Brien, M.; Duesberg, G.S. Controlled synthesis of transition metal dichalcogenide thin films for electronic applications. Appl. Surf. Sci. 2014, 297, 139–146. [Google Scholar] [CrossRef]
- Phung, V.-D.; Jung, W.-S.; Kim, J.-H.; Lee, S.-W. Gold nanostructures electrodeposited on graphene oxide-modified indium tin oxide glass as a surface-enhanced Raman scattering-active substrate for ultrasensitive detection of dopamine neurotransmitter. Jpn. J. Appl. Phys. 2018, 57, 08PF02. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Hur, J. MoS2-C-TiC Nanocomposites as New Anode Materials for High-Performance Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 996–1000. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Nguyen, Q.H.; Hur, J. High-Performance ZnTe-TiO2-C nanocomposite with half-cell and full-cell applications as promising anode material for Li-Ion batteries. Appl. Surf. Sci. 2020, 509, 144718. [Google Scholar] [CrossRef]
- Liu, N.; Choi, W.; Kim, H.; Jung, C.; Kim, J.; Choo, S.H.; Kwon, Y.; An, B.-S.; Hong, S.; So, S.; et al. Rapid and mass-producible synthesis of high-crystallinity MoSe2 nanosheets by ampoule-loaded chemical vapor deposition. Nanoscale 2020, 12, 6991–6999. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.-V.; Shim, K.; Vo Thi, T.-T.; Kook, J.-K.; An, S.S.A.; Lee, S.-W. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries? Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Zhang, C.; Park, S.-H.; Seral-Ascaso, A.; Barwich, S.; McEvoy, N.; Boland, C.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. High capacity silicon anodes enabled by MXene viscous aqueous ink. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K.; Pradhan, D. Microwave-Assisted Greener Synthesis of Defect-Rich Tungsten Oxide Nanowires with Enhanced Photocatalytic and Photoelectrochemical Performance. J. Phys. Chem. C 2018, 122, 3183–3193. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Emin, S.; Altinkaya, C.; Semerci, A.; Okuyucu, H.; Yildiz, A.; Stefanov, P. Tungsten carbide electrocatalysts prepared from metallic tungsten nanoparticles for efficient hydrogen evolution. Appl. Catal. B 2018, 236, 147–153. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.; Ko, M.J.; Lee, D.-K.; Kim, H.; Kim, S.H.; Kim, K. Solution processed WO3 layer for the replacement of PEDOT:PSS layer in organic photovoltaic cells. Org. Electron. 2012, 13, 959–968. [Google Scholar] [CrossRef]
- Li, W.-J.; Fu, Z.-W. Nanostructured WO3 thin film as a new anode material for lithium-ion batteries. Appl. Surf. Sci. 2010, 256, 2447–2452. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chang, Y.-H.; Hsu, C.-L.; Wei, K.-H.; Chiang, C.-Y.; Li, L.-J. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 12302–12309. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; English, C.R.; Meng, F.; Forticaux, A.; Hamers, R.J.; Jin, S. Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ. Sci. 2014, 7, 2608–2613. [Google Scholar] [CrossRef]
- Chen, Z.; Gong, W.; Cong, S.; Wang, Z.; Song, G.; Pan, T.; Tang, X.; Chen, J.; Lu, W.; Zhao, Z. Eutectoid-Structured WC/W2C heterostructures: A new platform for long-term alkaline hydrogen evolution reaction at low overpotentials. Nano Energy 2020, 68, 104335. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Guo, Z.; Liu, H. Synthesis of tungsten disulfide (WS2) nanoflakes for lithium ion battery application. Electrochem. Commun. 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Srinivaas, M.; Wu, C.-Y.; Duh, J.-G.; Wu, J.M. Highly Rich 1T Metallic Phase of Few-Layered WS2 Nanoflowers for Enhanced Storage of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 10363–10370. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Hu, Q.; Zhou, J.; Feng, R.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Li, Y.; et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. First principles prediction of two-dimensional tungsten carbide (W2C) monolayer and its Li storage capability. Comput. Condens. Matter 2017, 10, 35–38. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Van Le, Q.; Kim, I.T. Strategy for controlling the morphology and work function of W2C/WS2 nanoflowers. J. Alloys Compd. 2020, 829, 154582. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, S.Y.; Lee, T.H.; Jang, H.W.; Le, Q.V.; Kim, I.T. Facile synthesis of W2C@WS2 alloy nanoflowers and their hydrogen generation performance. Appl. Surf. Sci. 2020, 504, 144389. [Google Scholar] [CrossRef]
- Rout, C.S.; Joshi, P.D.; Kashid, R.V.; Joag, D.S.; More, M.A.; Simbeck, A.J.; Washington, M.; Nayak, S.K.; Late, D.J. Superior field emission properties of layered WS2-RGO nanocomposites. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kurlov, A.S.; Gusev, A.I. Tungsten carbides and W-C phase diagram. Inorg. Mater. 2006, 42, 121–127. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Fei, Y.W.; Suzuki, A.; Ohtani, E.; Funakoshi, K. Pressure-Volume-Temperature equation of state of tungsten carbide to 32 GPa and 1673 K. J. Appl. Phys. 2010, 108, 053513. [Google Scholar] [CrossRef]
- Molina-Sánchez, A.; Wirtz, L. Phonons in single-layer and few-layer MoS2 and WS2. Phys. Rev. B 2011, 84, 155413. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Xia, B.; Qi, X.; Wang, H.; Xu, R.; Wang, J.-Y.; Zhang, H.; Wang, X. Nano-Tungsten carbide decorated graphene as co-catalysts for enhanced hydrogen evolution on molybdenum disulfide. Chem. Commun. 2013, 49, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Pang, K.; Wu, X.; Zhang, Z.; Xu, X.; Ren, J.; Huang, W.; Song, R. In Situ Hydrothermal Synthesis MoS2/Guar Gum Carbon Nanoflowers as Advanced Electrocatalysts for Electrocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 8688–8696. [Google Scholar] [CrossRef]
- Shiva, K.; Ramakrishna Matte, H.S.S.; Rajendra, H.B.; Bhattacharyya, A.J.; Rao, C.N.R. Employing synergistic interactions between few-layer WS2 and reduced graphene oxide to improve lithium storage, cyclability and rate capability of Li-ion batteries. Nano Energy 2013, 2, 787–793. [Google Scholar] [CrossRef]
- Mak, K.F.; He, K.; Lee, C.; Lee, G.H.; Hone, J.; Heinz, T.F.; Shan, J. Tightly bound trions inmonolayer MoS2. Nat. Mater. 2013, 12, 207–211. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Moya, A.A. Identification of characteristic time constants in the initial dynamic response of electric double layer capacitors from high-frequency electrochemical impedance. J. Power Sour. 2018, 397, 124–133. [Google Scholar] [CrossRef]
- Samad, A.; Shafique, A.; Kim, H.J.; Shin, Y.-H. Superionic and electronic conductivity in monolayer W2C: Ab initio predictions. J. Mater. Chem. A 2017, 5, 11094–11099. [Google Scholar] [CrossRef]







| Sample | Rs | Rct | RSEI |
|---|---|---|---|
| WS2 NFs | 2.12 | 627 | 430 |
| WC/WS2_2h | 3.04 | 381 | 367 |
| WC/WS2_4h | 1.24 | 312 | 350 |
| WC/WS2_12h | 5.41 | 620 | 422 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. https://doi.org/10.3390/nano10071336
Nguyen TP, Kim IT. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials. 2020; 10(7):1336. https://doi.org/10.3390/nano10071336
Chicago/Turabian StyleNguyen, Thang Phan, and Il Tae Kim. 2020. "W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage" Nanomaterials 10, no. 7: 1336. https://doi.org/10.3390/nano10071336
APA StyleNguyen, T. P., & Kim, I. T. (2020). W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials, 10(7), 1336. https://doi.org/10.3390/nano10071336