The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System
Abstract
:1. Introduction
2. Materials and Methods
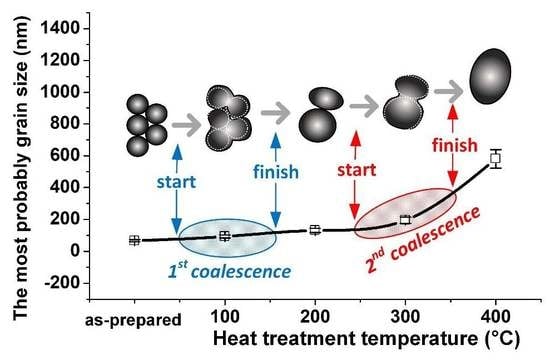
3. Results and Discussion
4. Conclusions
- It has been established that an increase in grain size with a simultaneous decrease in the number of grain boundaries leads to a decrease in the number of barriers to the distribution of dislocations during mechanical deformation. Consequently, the mechanical properties decrease with increasing grain size, especially in the volume of the material, since the surface as a whole is characterized by an incomparably greater defectiveness, and other mechanisms act because of this.
- Heat treatment in air activates surface oxidation. The oxidation process was studied step by step with increasing temperature while using an analysis of deformation curves. The thickness of the oxide layer increases from about 5 to 20 nm with an increasing temperature of heat treatment. It was found that when the oxide thickness hox is less than the indentation depth (hox < h), the hardness value includes two components: hardness of oxide and NiFe. This is true for the range from the as-prepared sample to the sample after treatment at 200 °C. Hardness increases from 8.6 to 11.0 GPa in this range. When hox > h, (after treatment at 300 and 400 °C), the hardness value remains constant at about 11.0 GPa, since the indentation takes place inside the oxide layer. It was also found that surface oxidation does not significantly affect the mechanical properties of the internal volume of the NiFe film.
- The third process, which is activated by heat treatment, is the diffusion of Au atoms from the sublayer into the NiFe film. Diffusing atoms are point defects, which facilitate the propagation of deformation and soften NiFe. The gold concentration for each temperature at the investigated indentation depth (10 and 50 nm) was calculated using I Fick’s law and the Arrhenius equation. Consequently, it was found that a relative concentration of more than 0.02 is a critical point, after passing through which a decrease in hardness, elastic modulus, and resistance to elastoplastic deformation begins.
Author Contributions
Funding
Conflicts of Interest
References
- McCrea, J.L.; Palumbo, G.; Hibbard, G.D.; Erb, U. Properties and applications for electrodeposited nanocrystalline Fe-Ni alloys. Rev. Adv. Mater. Sci. 2003, 5, 252–258. [Google Scholar]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings–A review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Koch, C.C. Structural nanocrystalline materials: An overview. J. Mater. Sci. 2007, 42, 1403–1414. [Google Scholar] [CrossRef]
- Erb, U.; Aust, K.T.; Palumbo, G. Electrodeposited nanocrystalline metals, alloys, and composites C.C. Koch Nanostructured Mater. In Nanostructured Materials (Second Edition) Processing, Properties, and Applications, 2nd ed.; William Andrew Inc.: Norwich, NY, USA, 2007; pp. 235–292. [Google Scholar]
- Dijith, K.S.; Aiswarya, R.; Praveen, M.; Pillai, S.; Surendran, K.P. Polyol derived Ni and NiFe alloys for effective shielding of electromagnetic interference. Mater. Chem. Front. 2018, 2, 1829–1841. [Google Scholar] [CrossRef]
- Evanczuk, S. Magnetic shielding materials to protect sensitive electronics. Electron. Prod. 2014, 56, 11. [Google Scholar]
- Park, J.; Lee, J.W.; Choi, H.J.; Jang, W.G.; Kim, T.S.; Suh, D.S.; Jeong, H.Y.; Chang, S.Y.; Roh, J.C.; Yoo, C.S.; et al. Electromagnetic interference shielding effectiveness of sputtered NiFe/Cu multi-layer thin film at high frequencies. Thin Solid Films 2019, 677, 130–136. [Google Scholar] [CrossRef]
- Nyunt, P.W.; Vlasik, K.F.; Grachev, V.M.; Dmitrenko, V.V.; Novikov, A.S.; Petrenko, D.V.; Ulin, S.E.; Uteshev, Z.M.; Chernysheva, I.V.; Shustov, A.E. Application Prospects of Multilayer Film Shields for Space Research Instrumentation. Phys. Procedia 2015, 74, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Nicolaescu, D.; Filip, V. Modelling of a magnetic sensor based on vacuum field emission. Appl. Surf. Sci. 1996, 94–95, 87–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, C.; Wang, Q.; Kong, Q.; Chen, G.; Guan, H.; Dong, C. Highly Sensitive and Selective Toluene Sensor of Bimetallic Ni/Fe-MOFs Derived Porous NiFe2O4 Nanorods. Ind. Eng. Chem. Res. 2019, 58, 9450–9457. [Google Scholar] [CrossRef]
- Chowdhury, P. Development of magnetoresistive thin film sensor for magnetic field sensing applications. In Proceedings of the 57th DAE Solid State Physics Symposium 2012 AIP Conference Proceedings, Mumbai, India, 3–7 December 2012; Volume 1512, pp. 30–33. [Google Scholar]
- Brian, R.A.; Shanahan, J.; Waldron, F.; Mathúna, C.O.; Rohan, J.F. Anisotropic Ni–Fe–B films with varying alloy composition for high frequency magnetics on silicon applications. Appl. Surf. Sci. 2015, 357, 385–390. [Google Scholar]
- Osaka, T.; Asahi, T.; Kawaji, J.; Yokoshima, T. Development of high-performance magnetic thin film for high-density magnetic recording. Electrochim. Acta 2005, 50, 4576–4585. [Google Scholar] [CrossRef]
- Grabchikov, S.S.; Trukhanov, A.V.; Trukhanova, S.V.; Kazakevich, I.S.; Solobaya, A.A.; Erofeenko, V.T.; Vasilenkov, N.A.; Volkova, O.S.; Shakine, A. Effectiveness of the magnetostatic shielding by the cylindrical shells. J. Magn. Magn. Mater. 2016, 398, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Trukhanov, A.V.; Grabchikov, S.S.; Solobai, A.A.; Tishkevich, D.I.; Trukhanov, S.V.; Trukhanova, E.L. AC and DC-shielding properties for the Ni80Fe20/Cu film structures. J. Magn. Magn. Mater. 2017, 443, 142–148. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corros. Sci. 2010, 66, 075005. [Google Scholar] [CrossRef]
- Kumar, D.V.; Ayyagari, S.; Prasad, M.J.N.V. Mechanical characteristics and electrochemical behaviour of electrodeposited nanocrystalline iron and iron-nickel alloy. Mater. Chem. Phys. 2017, 201, 26–34. [Google Scholar] [CrossRef]
- Giallonardo, J.D.; Erb, U.; Aust, K.T.; Palumbo, G. The influence of grain size and texture on the Young’s modulus of nanocrystalline nickel and nickel–iron alloys Philos. Mag. Abingdon (Abingdon) 2011, 91, 4594–4605. [Google Scholar] [CrossRef]
- Hadian, A.S.E.; Gabe, D.R. Residual stresses in electrodeposits of nickel and nickel–iron alloys. Surf. Coat. Technol. 1999, 122, 118–135. [Google Scholar] [CrossRef]
- Koo, B.; Yoo, B. Electrodeposition of low-stress NiFe thin films from a highly acidic electrolyte. Surf. Coat. Technol. 2010, 205, 740–744. [Google Scholar] [CrossRef]
- Dmitrievich, T.R. Normal Electrochemical Deposition of NiFe Films. Adv. Res. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Hoang, T.T.H.; Gewirth, A.A. High Activity Oxygen Evolution Reaction Catalysts from Additive Controlled Electrodeposited Ni and NiFe Films. ACS Catal. 2016, 6, 1159–1164. [Google Scholar] [CrossRef]
- Yanai, T.; Koda, K.; Eguchi, K.; Takashima, K.; Morimura, T.; Nakano, M.; Fukunaga, H. Effect of ammonium chloride in plating baths on soft magnetic properties of electroplated Fe-Ni films. IEEE Trans. Magn. 2017, 53, 2004303. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Koda, K.; Eguchi, K.; Morimura, T.; Takashima, K.; Nakano, M.; Fukunaga, H. Effect of an annealing on magnetic properties of Fe-Ni films electroplated in citric-acid-based plating baths. AIP Adv. 2018, 8, 047706. [Google Scholar] [CrossRef]
- Zhanga, N.; Jinb, S.B.; Shab, G.; Yua, J.K.; Caia, X.C.; Dua, C.C.; Shena, T.D. Segregation induced hardening in annealed nanocrystalline Ni-Fe alloy. Mater. Sci. Eng. A Struct. 2018, 735, 354–360. [Google Scholar] [CrossRef]
- Kotan, H.; Saber, M.; Koch, C.C.; Scattergood, R.O. Effect of annealing on microstructure, grain growth, and hardness of nanocrystalline Fe–Ni alloys prepared by mechanical alloying. Mater. Sci. Eng. A Struct. 2012, 552, 310–315. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Kwon, S.-K.; Lee, J.S. Annealing effect on microstructure and magnetic properties of flake-shaped agglomerates of Ni–20wt%Fe nanopowder. J. Alloys Compd. 2014, 613, 164–169. [Google Scholar] [CrossRef]
- Li, H.; Jiang, F.; Ni, S.; Li, L.; Sha, G.; Liao, X.; Ringer, S.P.; Choo, H.; Liawb, P.K.; Misra, A. Mechanical behaviors of as-deposited and annealed nanostructured Ni–Fe alloys. Scr. Mater. 2011, 65, 1–4. [Google Scholar] [CrossRef]
- Rusakova, V.S.; Kadyzhanovb, K.K.; Kozlovskiy, A.L.; Fadeeva, M.S.; Zdorovets, M.V. Phase transformations as a result of thermal annealing of nanocomposite Fe–Ni/Fe–Ni–O particles. Ceram. Int. 2020, 46, 1586–1595. [Google Scholar] [CrossRef]
- Kramer, D.E.; Yoder, K.B.; Gerberich, W.W. Surface constrained p1asticity: Oxide rupture and the yield point process. Philos. Mag. A 2001, 81, 2033–2058. [Google Scholar]
- Corcoran, S.G.; Colton, R.J.; Lilleodden, E.T.; Gerberich, W.W. Anoma1ous plastic deformation at surfaces: Nanoindentation of gold single crystals. Phys. Rev. B 1997, 55, R16057–R16060. [Google Scholar] [CrossRef]
- Li, J.; Van Vliet, K.J.; Zhu, T.; Yip, S.; Suresh, S. Atomistic mechanisms governing elastic limit and incipient p1asticity in crystals. Nature 2002, 418, 307–310. [Google Scholar] [CrossRef]
- Xia, J.; Li, C.X.; Dong, H.; Bell, T. Nanoindentation and nanoscratch properties of a thermal oxidation treated γ-TiAl based alloy. Surf. Coat. Technol. 2006, 200, 4755–4762. [Google Scholar] [CrossRef]
- Deng, G.Y.; Tieu, A.K.; Su, L.H.; Zhu, H.T.; Zhu, Q.; Zamri, W.F.H.; Kong, C. Characterizing deformation behaviour of an oxidized high speed steel: Effects of nanoindentation depth, friction and oxide scale porosity. Int. J. Mech. Sci. 2019, 155, 267–285. [Google Scholar] [CrossRef]
- Lu, Q.H.; Huang, R.; Wang, L.S.; Wu, Z.G.; Li, C.; Luo, Q.; Zuo, S.Y.; Li, J.; Peng, D.L.; Han, G.L.; et al. Thermal annealing and magnetic anisotropy of NiFe thin films on n-Si for spintronic device applications. J. Magn. Magn. Mater. 2015, 394, 253–259. [Google Scholar] [CrossRef]
- Zubar, T.; Trukhanov, A.; Vinnik, D.; Astapovich, K.; Tishkevich, D.; Kaniukov, E.; Kozlovskiy, A.; Zdorovets, M.; Trukhanov, S. Features of the growth processes and magnetic domain structure of nife nano-objects. J. Phys. Chem. C 2019, 123, 26957–26964. [Google Scholar] [CrossRef]
- Zubar, T.I.; Fedosyuk, V.M.; Trukhanov, A.V.; Kovaleva, N.N.; Astapovich, K.A.; Vinnik, D.A.; Trukhanova, E.L.; Kozlovskiy, A.L.; Zdorovets, M.V.; Solobai, A.A.; et al. Control of Growth Mechanism of Electrodeposited Nanocrystalline NiFe Films. J. Electrochem. Soc. 2019, 166, D173–D180. [Google Scholar] [CrossRef]
- Zubar, T.I.; Panina, L.V.; Kovaleva, N.N.; Sharko, S.A.; Tishkevich, D.I.; Vinnik, D.A.; Gudkova, S.A.; Trukhanova, E.L.; Trofimov, E.A.; Chizhik, S.A.; et al. Anomalies in growth of electrodeposited Ni–Fe nanogranular films. CrystEngComm 2018, 20, 2306–2315. [Google Scholar] [CrossRef]
- Ge, W.; He, T.; Wang, M.; Li, J. Nano-Grain Ni/ZrO2 Functional Gradient Coating Fabricated by Double Pulses Electrodeposition with Enhanced High Temperature Corrosion Performance. Coatings 2020, 10, 332. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.S.; Sergelius, P.; Zierold, R.; Moreno, J.M.M.; Görlitz, D.; Nielsch, K. Magnetic characterization of nickel-rich NiFe nanowires grown by pulsed electrodeposition. J. Mater. Chem. 2012, 22, 8549–8557. [Google Scholar] [CrossRef]
- Zubar, T.I.; Sharko, S.A.; Tishkevich, D.I.; Kovaleva, N.N.; Vinnik, D.A.; Gudkova, S.A.; Trukhanova, E.L.; Trofimov, E.A.; Chizhik, S.A.; Panina, L.V.; et al. Anomalies in Ni-Fe nanogranular films growth. J. Alloys Compd. 2018, 748, 970–978. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Lupicka, O.; Kuprin, A.S.; Tolmachova, G.N.; Ovcharenko, V.D.; Kolodiy, I.V.; Sawczak, M.; Kochmanska, A.E.; Kochmanski, P. Structure of CrON coatings formed in vacuum arc plasma fluxes. Surf. Coat. Technol. 2016, 309, 920–930. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Zubar, T.; Chizhik, S.; Gilewicz, A.; Lupicka, O.; Warcholinski, B. Surface Microstructure of Mo(C)N Coatings Investigated by AFM. J. Mater. Eng. Perform. 2016, 25, 5450–5459. [Google Scholar] [CrossRef] [Green Version]
- Warcholinski, B.; Gilewicz, A.; Kuprin, A.S.; Tolmachova, G.N.; Ovcharenko, V.D.; Kuznetsova, T.A.; Zubar, T.I.; Khudoley, A.L.; Chizhik, S.A. Mechanical properties of Cr-O-N coatings deposited by cathodic arc evaporation. Vacuum 2018, 156, 97–107. [Google Scholar] [CrossRef]
- Hwang, Y.-M.; Pan, C.-T.; Lu, Y.-X.; Jian, S.-R.; Chang, H.-W.; Juang, J.-Y. Influence of Post-Annealing on the Structural and Nanomechanical Properties of Co Thin Films. Micromachines 2020, 11, 180. [Google Scholar] [CrossRef] [Green Version]
- Zavaleyev, V.; Walkowicz, J.; Kuznetsova, T.; Zubar, T. The dependence of the structure and mechanical properties of thin ta-C coatings deposited using electromagnetic Venetian blind plasma filter on their thickness. Thin Solid Films 2017, 638, 153–158. [Google Scholar] [CrossRef]
- Oliver, W.C. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to metrology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Gorban, A.N.; Sargsyan, H.P.; Wahab, H.A. Quasichemical Models of Multicomponent Nonlinear Diffusion. Math. Model. Nat. Phenom. 2011, 6, 184–262. [Google Scholar] [CrossRef] [Green Version]
- Le Claire, A.D. The theory of D0 in the Arrhenius equation for self-diffusion in cubic metals. Acta Metall. 1953, 1, 438–447. [Google Scholar] [CrossRef]
- Shujahadeen, M.H.H.; Azizbc, B.; Azhaa, M.A.S.; Azlia, A.A.; Shukurd, M.F.; Yusofe, Y.M.; Ninie, S.K.M.; Manang, S.A.; Kadir, M.F.Z. Solid-state double layer capacitors and protonic cell fabricated with dextran from Leuconostoc mesenteroides based green polymer electrolyte. Mater. Chem. Phys. 2020, 241, 122290. [Google Scholar]
- Ju, H.; Ding, N.; Xu, J.; Yu, L.; Asempah, I.; Xu, J.; Yi, G.; Ma, B. Crystal structure and the improvement of the mechanical and tribological properties of tungsten nitride films by addition of titanium. Surf. Coat. Technol. 2018, 345, 132–139. [Google Scholar] [CrossRef]
- Meng, Q.; Malinovskis, P.; Nedfors, N.; Mao, F.; Andersson, M.; Sun, Y.; Jansson, U. Characterization of amorphous Zr–Si–C thin films deposited by DC magnetron sputtering. Surf. Coat. Technol. 2015, 261, 227–234. [Google Scholar] [CrossRef]
- Bagdasaryan, A.A.; Pshyk, A.V.; Coy, L.E.; Konarski, P.; Misnik, M.; Ivashchenko, V.I.; Kempiński, M.; Mediukh, N.R.; Pogrebnjak, A.D.; Beresnev, V.M.; et al. A new type of (TiZrNbTaHf)N/MoN nanocomposite coating: Microstructure and properties depending on energy of incident ions. Compos. Part B Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Soer, W.A.; Aifantis, K.E.; De Hosson, J.M. Incipient plasticity during nanoindentation at grain boundaries in body-centered cublc metals. Acta Mater. 2005, 53, 4665–4676. [Google Scholar] [CrossRef] [Green Version]
- Pergande, S.R.; Polycarpou, A.A.; Conry, T.F. Nanomechanical Properties of Aluminum 390-T6 Rough Surfaces Undergoing Tribological Testing. J. Tribol. 2004, 126, 573–582. [Google Scholar] [CrossRef]





| Parameter | Value | |
|---|---|---|
| Electrolyte composition, g/L | NiSO4 | 210 |
| NiCl2 | 20 | |
| H3BO3 | 30 | |
| MgSO4 | 60 | |
| FeSO4 | 15 | |
| Saccharin | 1 | |
| Electrolyte pH | 2.3–2.5 | |
| Electrolyte temperature, °C | 30–33 | |
| Anodes | Ni | |
| Current | pulsed | |
| Pulse duration, s | 10−3 | |
| Pause duration, s | 10−3 | |
| Current density, mA/cm2 | 25 | |
| Deposition time, s | 300 | |
| Effective deposition time, s | 150 | |
| Parameter | Value |
|---|---|
| Substrate | Si wafer (100) |
| Thickness of Au layer, nm | 100 |
| Thickness of NiFe layer, nm | 600 |
| Fe content, at.% | 24.45 |
| Ni content, at.% | 75.55 |
| Type of crystal structure (NiFe) | cubic face-centered |
| Space group | Fm3m (No. 225) |
| Unit cell parameter, Å | 3.573 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubar, T.; Fedosyuk, V.; Tishkevich, D.; Kanafyev, O.; Astapovich, K.; Kozlovskiy, A.; Zdorovets, M.; Vinnik, D.; Gudkova, S.; Kaniukov, E.; et al. The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System. Nanomaterials 2020, 10, 1077. https://doi.org/10.3390/nano10061077
Zubar T, Fedosyuk V, Tishkevich D, Kanafyev O, Astapovich K, Kozlovskiy A, Zdorovets M, Vinnik D, Gudkova S, Kaniukov E, et al. The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System. Nanomaterials. 2020; 10(6):1077. https://doi.org/10.3390/nano10061077
Chicago/Turabian StyleZubar, Tatiana, Valery Fedosyuk, Daria Tishkevich, Oleg Kanafyev, Ksenia Astapovich, Artem Kozlovskiy, Maxim Zdorovets, Denis Vinnik, Svetlana Gudkova, Egor Kaniukov, and et al. 2020. "The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System" Nanomaterials 10, no. 6: 1077. https://doi.org/10.3390/nano10061077
APA StyleZubar, T., Fedosyuk, V., Tishkevich, D., Kanafyev, O., Astapovich, K., Kozlovskiy, A., Zdorovets, M., Vinnik, D., Gudkova, S., Kaniukov, E., Sombra, A. S. B., Zhou, D., Jotania, R. B., Singh, C., Trukhanov, S., & Trukhanov, A. (2020). The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System. Nanomaterials, 10(6), 1077. https://doi.org/10.3390/nano10061077