Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Polarization Measurements
3.2. Chemical Composition Study
3.3. Deposition Rate Determination
3.4. Surface Morphology Investigation
3.5. Average Grain Size Analysis
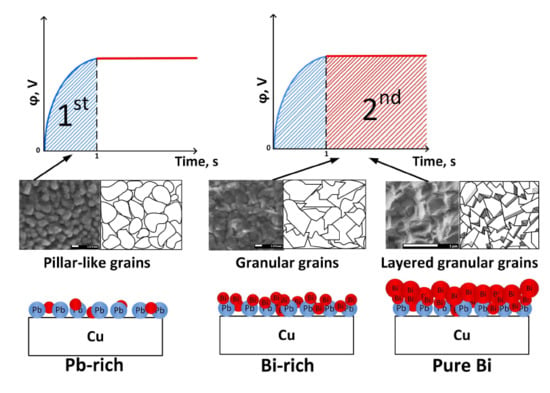
3.6. Growth Mechanism Explanation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ziegler, J.P. Status of reversible electrodeposition electrochromic devices. Sol. Energy Mater. Sol. Cells 1999, 56, 477–493. [Google Scholar] [CrossRef]
- Córdoba De Torresi, S.I.; Carlos, I.A. Optical characterization of bismuth reversible electrodeposition. J. Electroanal. Chem. 1996, 414, 11–16. [Google Scholar] [CrossRef]
- Rehacek, V.; Hotovy, I.; Vojs, M. Bismuth film voltammetric sensor on pyrolyzed photoresist/alumina support for determination of heavy metals. Electroanalysis 2014, 26, 898–903. [Google Scholar] [CrossRef]
- Yao, J.D.; Shao, J.M.; Yang, G.W. Ultra-broadband and high-responsive photodetectors based on bismuth film at room temperature. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Moral-Vico, J.; Casañ-Pastor, N.; Camón, A.; Pobes, C.; Jáudenes, R.M.; Strichovanec, P.; Fàbrega, L. Microstructure and electrical transport in electrodeposited Bi films. J. Electroanal. Chem. 2019, 832, 40–47. [Google Scholar] [CrossRef]
- Hofmann, P. The surfaces of bismuth: Structural and electronic properties. Prog. Surf. Sci. 2006, 81, 191–245. [Google Scholar] [CrossRef]
- Yang, F.; Miao, L.; Wang, Z.F.; Yao, M.Y.; Zhu, F.; Song, Y.R.; Wang, M.X.; Xu, J.P.; Fedorov, A.V.; Sun, Z.; et al. Spatial and energy distribution of topological edge states in single Bi(111) bilayer. Phys. Rev. Lett. 2012, 109, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sabater, C.; Gosálbez-Martínez, D.; Fernández-Rossier, J.; Rodrigo, J.G.; Untiedt, C.; Palacios, J.J. Topologically protected quantum transport in locally exfoliated bismuth at room temperature. Phys. Rev. Lett. 2013, 110, 1–5. [Google Scholar] [CrossRef]
- O’Brien, B.; Plaza, M.; Zhu, L.Y.; Perez, L.; Chien, C.L.; Searson, P.C. Magnetotransport properties of electrodeposited bismuth films. J. Phys. Chem. C 2008, 112, 12018–12023. [Google Scholar] [CrossRef]
- Švancara, I.; Prior, C.; Hočevar, S.B.; Wang, J. A decade with bismuth-based electrodes in electroanalysis. Electroanalysis 2010, 22, 1405–1420. [Google Scholar] [CrossRef]
- Baldrianova, L.; Svancara, I.; Sotiropoulos, S. Anodic stripping voltammetry at a new type of disposable bismuth-plated carbon paste mini-electrodes. Anal. Chim. Acta 2007, 599, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Margaroni, D. World energy consumption. Ind. Lubr. Tribol. 1998, 50, 312–317. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Le Ninivin, C.; Mabic, S.; Dimitrakopoulos, T. Electrochemical sensor for trace silicic acid detection based on bismuth film electrode. J. Electroanal. Chem. 2011, 651, 46–49. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, K.; Bai, D.; Zhu, Z. Synthesis and electrochemical sensing toward heavy metals of bunch-like bismuth nanostructures. Nanoscale Res. Lett. 2010, 5, 398–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Formation and corrosion behavior of nickel/alumina nanocomposites. Solid State Phenom. 2020, 299, 100–106. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Li, G.; Zhang, L. A route to fabricate single crystalline bismuth nanowire arrays with different diameters. Chem. Phys. Lett. 2003, 378, 244–249. [Google Scholar] [CrossRef]
- Yang, F.Y.; Liu, K.; Hong, K.; Reich, D.H.; Searson, P.C.; Chien, C.L. Large magnetoresistance of electrodeposited single-crystal bismuth thin films. Science 1999, 284, 1335–1337. [Google Scholar] [CrossRef] [Green Version]
- Iyomoto, N.; Sadleir, J.E.; Figueroa-feliciano, E.; Saab, T.; Bandler, S.; Kilbourne, C.; Chervenak, J.; Talley, D.; Finkbeiner, R.; Brekosky, R.; et al. Optimization of X-ray absorbers for TES microcalorimeters. In High-Energy Detectors in Astronomy; SPIE: Bellingham, WA, USA, 2004; Volume 5501, pp. 145–154. [Google Scholar]
- Yoshino, T.; Yoshida, K.; Hagihara, T.; Sato, K.; Morita, U.; Ishisaki, Y.; Fujimoto, R.; Yamasaki, N.Y.; Mitsuda, K. Properties of vacuum-evaporated bismuth absorber. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers, Detect. Assoc. Equip. 2006, 559, 432–435. [Google Scholar] [CrossRef]
- Brown, A.D.; Bandler, S.R.; Brekosky, R.; Chervenak, J.A.; Figueroa-Feliciano, E.; Finkbeiner, F.; Iyomoto, N.; Kelley, R.L.; Kilbourne, C.A.; Porter, F.S.; et al. Absorber materials for transition-edge sensor X-ray microcalorimeters. J. Low Temp. Phys. 2008, 151, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Gades, L.M.; Cecil, T.W.; Divan, R.; Schmidt, D.R.; Ullom, J.N.; Madden, T.J.; Yan, D.; Miceli, A. Development of Thick Electroplated Bismuth Absorbers for Large Collection Area Hard X-ray Transition Edge Sensors. IEEE Trans. Appl. Supercond. 2017, 27, 1–5. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Zubar, T.I.; Vasin, D.S.; Trukhanov, A.V. Correlation of the synthesis conditions and microstructure for Bi-based electron shields production. J. Alloys Compd. 2018, 749, 1036–1042. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Zubar, T.I.; Vasin, D.S.; Trukhanov, A.V.; Kozlovskiy, A.L.; Zdorovets, M.M. Effect of the Synthesis Conditions and Microstructure for Highly Effective Electron Shields Production Based on Bi Coatings. ACS Appl. Energy Mater. 2018, 1, 1695–1702. [Google Scholar] [CrossRef]
- Yasaka, P.; Pattanaboonmee, N.; Kim, H.J.; Limkitjaroenporn, P.; Kaewkhao, J. Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Maghrabi, H.A.; Vijayan, A.; Deb, P.; Wang, L. Bismuth oxide-coated fabrics for X-ray shielding. Text. Res. J. 2016, 86, 649–658. [Google Scholar] [CrossRef]
- McCaffrey, J.P.; Mainegra-Hing, E.; Shen, H. Optimizing non-Pb radiation shielding materials using bilayers. Med. Phys. 2009, 36, 5586–5594. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Lu, Z.; Zhao, H.; Yang, H.; Chen, R. Photoinduced switchable wettability of bismuth coating with hierarchical dendritic structure between superhydrophobicity and superhydrophilicity. Appl. Surf. Sci. 2015, 353, 735–743. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Pu, F.; Yin, X.; Xia, Y.; Huang, W.; Li, Z. Facile fabrication of superhydrophobic Bi/Bi2O3 surfaces with hierarchical micro-nanostructures by electroless deposition or electrodeposition. Appl. Surf. Sci. 2014, 288, 558–563. [Google Scholar] [CrossRef]
- Duarte, M.M.E.; Pilla, A.S.; Sieben, J.M.; Mayer, C.E. Platinum particles electrodeposition on carbon substrates. Electrochem. Commun. 2006, 8, 159–164. [Google Scholar] [CrossRef]
- Mahalingam, T.; John, V.S.; Rajendran, S.; Sebastian, P.J. Electrochemical deposition of ZnTe thin films. Semicond. Sci. Technol. 2002, 17, 465–470. [Google Scholar] [CrossRef]
- Liu, L.; Qi, L.; Han, R.; Zhang, H.; Wang, Y.; Sun, H. Growth mechanism and magnetic properties of dendritic nanostructure prepared by pulse electrodeposition. J. Alloys Compd. 2017, 694, 1239–1245. [Google Scholar] [CrossRef]
- Da Tsai, Y.; Lien, C.H.; Hu, C.C. Effects of polyethylene glycol and gelatin on the crystal size, morphology, and Sn2+-sensing ability of bismuth deposits. Electrochim. Acta 2011, 56, 7615–7621. [Google Scholar] [CrossRef]
- Som, T.; Simo, A.; Fenger, R.; Troppenz, G.V.; Bansen, R.; Pfänder, N.; Emmerling, F.; Rappich, J.; Boeck, T.; Rademann, K. Bismuth hexagons: Facile mass synthesis, stability and applications. ChemPhysChem 2012, 13, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, W.; Lee, W. Bismuth nanowire thermoelectrics. J. Mater. Chem. C 2015, 3, 11999–12013. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Vinnik, D.A. Template assisted ni nanowires fabrication. Mater. Sci. Forum 2019, 946, 235–241. [Google Scholar] [CrossRef]
- Roh, J.W.; Ham, J.; Kim, J.; Moon, H.; Kim, H.S.; Lee, W. Extreme reduction of thermal conductivity by embedding Al2O3 nanoparticles into single-crystalline Bi nanowires. Acta Mater. 2017, 136, 315–322. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Vorobjova, A.I.; Trukhanov, A.V. Thermal stability of nano-crystalline nickel electrodeposited into porous alumina. Solid State Phenom. 2020, 299, 281–286. [Google Scholar] [CrossRef]
- Yakimchuk, D.V.; Khubezhov, S.A.; Bundyukova, V.D.; Kozlovskiy, A.L.; Zdorovets, M.V.; Shlimas, D.I.; Tishkevich, D.I.; Kaniukov, E.Y. Copper nanostructures into pores of SiO2/Si template: Galvanic displacement, chemical and structural characterization. Mater. Res. Express 2019, 6, 105058. [Google Scholar] [CrossRef]
- Vorobjova, A.I.; Shimanovich, D.L.; Sycheva, O.A.; Ezovitova, T.I.; Tishkevich, D.I.; Trykhanov, A.V. Studying the Thermodynamic Properties of Composite Magnetic Material Based on Anodic Alumina. Russ. Microelectron. 2019, 48, 107–118. [Google Scholar] [CrossRef]
- Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zdorovets, M.; Kozlovskiy, A.; Zubar, T.; Vinnik, D.; Dong, M.; Trukhanov, S.; Trukhanov, A.; et al. Electrochemical behaviour of Ti/Al2O3/Ni nanocomposite material in artificial physiological solution: Prospects for biomedical application. Nanomaterials 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Sandnes, E.; Williams, M.E.; Bertocci, U.; Vaudin, M.D.; Stafford, G.R. Electrodeposition of bismuth from nitric acid electrolyte. Electrochim. Acta 2007, 52, 6221–6228. [Google Scholar] [CrossRef]
- Vereecken, P.M.; Rodbell, K.; Ji, C.; Searson, P.C. Electrodeposition of bismuth thin films on n-GaAs (110). Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Bilican, D.; Fornell, J.; Sort, J.; Pellicer, E. Electrochemical synthesis of bismuth particles: Tuning particle shape through substrate type within a narrow potential window. Materials (Basel). 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, S.L.; Wei, X.; Chen, W.; Yao, C.; Gao, W. Microstructures and properties of electrodeposited Cu-Bi composite coatings. Int. J. Electrochem. Sci. 2014, 9, 2266–2277. [Google Scholar]
- Wada, H.; Nishisaka, Y.; Ichino, R.; Okido, M. Bi-Cu film deposition in aqueous solutions. Trans. Nonferrous Met. Soc. China (English Ed.) 2009, 19, 791–794. [Google Scholar] [CrossRef]
- Yang, M.; Hu, Z. Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J. Electroanal. Chem. 2005, 583, 46–55. [Google Scholar] [CrossRef]
- Perelygin, Y.P.; Kireev, S.Y.; Kireev, A.Y. BRIEF Electrodeposition of Bismuth from Acid Lactate Electrolyte. Russ. J. Appl. Chem. 2006, 79, 1200–1201. [Google Scholar] [CrossRef]
- Agapescu, C.; Cojocaru, A.; Cotarta, A.; Visan, T. Electrodeposition of bismuth, tellurium, and bismuth telluride thin films from choline chloride-oxalic acid ionic liquid. J. Appl. Electrochem. 2013, 43, 309–321. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, Y.H.; Luo, F.; Du, N.; Yan, C.H. Synthesis of bismuth with various morphologies by electrodeposition. Inorg. Chem. Commun. 2003, 6, 781–785. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, Y.; Zhang, H.; Jia, Y.; Zhang, J.; Li, C. Nucleation and growth of bismuth electrodeposition from alkaline electrolyte. Bull. Korean Chem. Soc. 2012, 33, 1541–1546. [Google Scholar] [CrossRef] [Green Version]
- Harbaugh, M.; Mathers, F.C. The Electrodeposition of Bismuth from Perchloric Acid Solutions. Trans. Electrochem. Soc. 1933, 64, 293. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Tsybulskaya, L.S.; Shendyukov, V.S.; Perevoznikov, S.S.; Trukhanov, S.V.; Trukhanova, E.L.; Trukhanov, A.V.; Vinnik, D.A. Electrochemical deposition regimes and critical influence of organic additives on the structure of Bi films. J. Alloys Compd. 2018, 735, 1943–1948. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Grabchikova, E.A.; Vasin, D.S.; Lastovskii, S.B.; Yakushevich, A.S.; Vinnik, D.A.; Zubar, T.I.; Kalagin, I.V.; Mitrofanov, S.V.; et al. Modeling of paths and energy losses of high-energy ions in single-layered and multilayered materials. Proc. Natl. Acad. Sci. Belarus, Phys. IOP Conf. Ser. Mater. Sci. Eng. 2020, 848, 012089. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Kozlovskiy, A.L.; Ryskulov, A.E.; Uglov, V.V.; Kislitsin, S.B.; Zdorovets, M.V.; Trukhanov, S.V.; Zubar, T.I.; Astapovich, K.A.; Tishkevich, D.I. Control of structural parameters and thermal conductivity of BeO ceramics using heavy ion irradiation and post-radiation annealing. Ceram. Int. 2019, 45, 15412–15416. [Google Scholar] [CrossRef]
- Tishkevich, D.I.; Grabchikov, S.S.; Lastovskii, S.B.; Trukhanov, S.V.; Vasin, D.S.; Zubar, T.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Sivakov, V.A.; Muradyan, T.R.; et al. Function composites materials for shielding applications: Correlation between phase separation and attenuation properties. J. Alloys Compd. 2019, 771, 238–245. [Google Scholar] [CrossRef]
- Zubar, T.I.; Sharko, S.A.; Tishkevich, D.I.; Kovaleva, N.N.; Vinnik, D.A.; Gudkova, S.A.; Trukhanova, E.L.; Trofimov, E.A.; Chizhik, S.A.; Panina, L.V.; et al. Anomalies in Ni-Fe nanogranular films growth. J. Alloys Compd. 2018, 748, 970–978. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Metals and Minerals: U.S. Geological Survey Minerals Yearbook; U.S. Geological Survey: Reston, VA, USA, 2011; Volume 1, 1101.
- Lotfian, S.; Mayer, C.; Chawla, N.; Llorca, J.; Misra, A.; Baldwin, J.K.; Molina-Aldareguía, J.M. Effect of layer thickness on the high temperature mechanical properties of Al/SiC nanolaminates. Thin Solid Films 2014, 571, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Zubar, T.; Trukhanov, A.; Vinnik, D.; Astapovich, K.; Tishkevich, D.; Kaniukov, E.; Kozlovskiy, A.; Zdorovets, M.; Trukhanov, S. Features of the Growth Processes and Magnetic Domain Structure of NiFe Nano-objects. J. Phys. Chem. C 2019, 123, 26957–26964. [Google Scholar] [CrossRef]
- Zubar, T.I.; Fedosyuk, V.M.; Trukhanov, A.V.; Kovaleva, N.N.; Astapovich, K.A.; Vinnik, D.A.; Trukhanova, E.L.; Kozlovskiy, A.L.; Zdorovets, M.V.; Solobai, A.A.; et al. Control of Growth Mechanism of Electrodeposited Nanocrystalline NiFe Films. J. Electrochem. Soc. 2019, 166, D173–D180. [Google Scholar] [CrossRef]
- Rahman, I.Z.; Khaddem-Mousavi, M.V.; Gandhi, A.A.; Lynch, T.F.; Rahman, M.A. Growth of electrodeposited Ni-Co and Fe-Co magnetic films on Cu substrates. J. Phys. Conf. Ser. 2007, 61, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Arafat, Y.; Sultana, S.T.; Dutta, I.; Panat, R. Effect of Additives on the Microstructure of Electroplated Tin Films. J. Electrochem. Soc. 2018, 165, D816–D824. [Google Scholar] [CrossRef] [Green Version]
- Almessiere, M.A.; Trukhanov, A.V.; Khan, F.A.; Slimani, Y.; Tashkandi, N.; Turchenko, V.A.; Zubar, T.I.; Tishkevich, D.I.; Trukhanov, S.V.; Panina, L.V.; et al. Correlation between microstructure parameters and anti-cancer activity of the [Mn0.5Zn0.5](EuxNdxFe2-2x)O4 nanoferrites produced by modified sol-gel and ultrasonic methods. Ceram. Int. 2020, 46, 7346–7354. [Google Scholar] [CrossRef]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page) (accessed on 6 June 2020).









| Series | Sample No. | Current Density (Dc), mA/cm2 | Deposition Duration (t), s | Deposition Rate (v), ~nm/s | Thickness (h), nm | Composition of Pb:Bi (C), % | Porosity (p), % | Average Grain Size, nm |
|---|---|---|---|---|---|---|---|---|
| 1 | 1_10 | 10 | 1 | 15 | 15 | 84.5:15.5 | 12.5 | 192 |
| 2_10 | 5 | 50 | 252 | 0.9:99.1 | 11.5 | 196 | ||
| 3_10 | 15 | 105 | 1575 | 0:100 | 8.4 | 222 | ||
| 4_10 | 30 | 165 | 4950 | 0:100 | 2.9 | 241 | ||
| 2 | 1_15 | 15 | 1 | 28 | 28 | 83.3:16.7 | 10.6 | 197 |
| 2_15 | 5 | 66 | 329 | 1.4:98.6 | 6.7 | 199 | ||
| 3_15 | 15 | 164 | 2465 | 0:100 | 5.0 | 272 | ||
| 4_15 | 30 | 274 | 8223 | 0:100 | 1.8 | 316 | ||
| 3 | 1_20 | 20 | 1 | 30 | 30 | 82.9:17.1 | 8.4 | 201 |
| 2_20 | 5 | 99 | 445 | 1.2:98.8 | 4.8 | 207 | ||
| 3_20 | 15 | 247 | 3704 | 0:100 | 3.0 | 335 | ||
| 4_20 | 30 | 329 | 9879 | 0:100 | 0.9 | 386 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tishkevich, D.; Grabchikov, S.; Zubar, T.; Vasin, D.; Trukhanov, S.; Vorobjova, A.; Yakimchuk, D.; Kozlovskiy, A.; Zdorovets, M.; Giniyatova, S.; et al. Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte. Nanomaterials 2020, 10, 1245. https://doi.org/10.3390/nano10061245
Tishkevich D, Grabchikov S, Zubar T, Vasin D, Trukhanov S, Vorobjova A, Yakimchuk D, Kozlovskiy A, Zdorovets M, Giniyatova S, et al. Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte. Nanomaterials. 2020; 10(6):1245. https://doi.org/10.3390/nano10061245
Chicago/Turabian StyleTishkevich, Daria, Sergey Grabchikov, Tatiana Zubar, Denis Vasin, Sergei Trukhanov, Alla Vorobjova, Dmitry Yakimchuk, Artem Kozlovskiy, Maxim Zdorovets, Sholpan Giniyatova, and et al. 2020. "Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte" Nanomaterials 10, no. 6: 1245. https://doi.org/10.3390/nano10061245
APA StyleTishkevich, D., Grabchikov, S., Zubar, T., Vasin, D., Trukhanov, S., Vorobjova, A., Yakimchuk, D., Kozlovskiy, A., Zdorovets, M., Giniyatova, S., Shimanovich, D., Lyakhov, D., Michels, D., Dong, M., Gudkova, S., & Trukhanov, A. (2020). Early-Stage Growth Mechanism and Synthesis Conditions-Dependent Morphology of Nanocrystalline Bi Films Electrodeposited from Perchlorate Electrolyte. Nanomaterials, 10(6), 1245. https://doi.org/10.3390/nano10061245