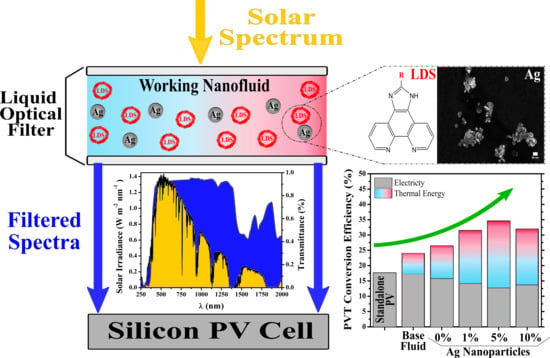
Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasmonic-Enhanced Luminescent Down-Shifting (PLDS) Nanofluid Preparation
2.3. PLDS Nanofluid Characterisation
2.3.1. Optical Transmittance of PLDS Filters
2.3.2. Fluorescence of PLDS Filters
2.3.3. Performance Evaluation of PLDS Liquid Filters
2.3.4. Photo-Thermal Conversion Efficiency of PLDS Filters
2.4. Merit Function of Spectral Beam-Splitting Photovoltaic-Thermal (SBS-PVT) System with PLDS Liquid Optical Filters
3. Results and Discussion
3.1. Optical Transmittance and Fluorescence of PLDS Nanofluid Filters
3.2. Stagnation Temperature and Photothermal Conversion Efficiency (PTE) of PLDS Nanofluid Filters
3.3. Performance of Concentrator Photovoltaic (C-PV) Solar Cell with PLDS Nanofluid Filters
3.4. Conversion Efficiency of SBS-PVT System and Merit Function with PLDS Nanofluids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IRENA Global Renewables Outlook: Energy Transformation 2050; International Renewable Energy Agency (IRENA): Abu Dhabi, UAE, 2020; ISBN 9789292602383. Available online: https://www.irena.org/publications/2020/Apr/Global-Renewables-Outlook-2020 (accessed on 19 June 2020).
- IEA Renewables: Heat. Available online: https://www.iea.org/reports/renewables-2019/heat (accessed on 7 June 2020).
- European Comission. Heating and Cooling: Fact and Figures. Available online: https://ec.europa.eu/energy/topics/energy-efficiency/heating-and-cooling_en#facts-and-figures (accessed on 11 June 2020).
- The European Power Sector in 2018 Up-To-Date Analysis on the Electriciy Transition. Agora Energiewend. 2019, p. 44. Available online: https://www.agora-energiewende.de/en/publications/the-european-power-sector-in-2018/ (accessed on 19 June 2020).
- Gayathri, P.; Harold, A. Future of Solar Photovoltaic. Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects; International Renewable Energy Agency (IRENA): Abu Dhabi, UAE, 2019; ISBN 978-92-9260-155-3. [Google Scholar]
- IRENA Renewable Power Generation Costs in 2019; International Renewable Energy Agency (IRENA): Abu Dhabi, UAE, 2019; ISBN 9789292602444.
- Markvart, T.; Castañer, L. Principles of Solar Cell Operation; McEvoy, A., Castañer, L., Markvart, T.B.T.-S.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–25. ISBN 978-0-12-386964-7. [Google Scholar]
- El Amin, A.A.; Al-Maghrabi, M.A. The Analysis of Temperature Effect for mc-Si Photovoltaic Cells Performance. Silicon 2018, 10, 1551–1555. [Google Scholar] [CrossRef]
- Wan, A.; Azman, C.M.; Engku, A.; Spenkuch, J.L.; Wahab, A.R.A.; Lewis, M.K.; Hassan, M.K.; Shariff, K.; Syariah, D.I.A.; Schmidt, U.; et al. Exergetic advancement of photovoltaic/thermal systems (PV/T): A review. J. Pengur. 2004, 23, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Dalezios, N.; Adamowski, K. Photovoltaic-thermal (PV/T) technology: A comprehensive review on applications and its advancement. Int. J. Energy Environ. Eng. 2017, 553–568. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazik, A.S.; Al-Sulaiman, F.A.; Saidur, R.; Ben-Mansour, R. A review on recent development for the design and packaging of hybrid photovoltaic/thermal (PV/T) solar systems. Renew. Sustain. Energy Rev. 2018, 95, 110–129. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Guo, F.-H.; Xiao, L. A Review on the Methodology for Calculating Heat and Exergy Losses of a Conventional Solar PV/T System. Int. J. Green Energy 2015, 12, 379–397. [Google Scholar] [CrossRef]
- Hjerrild, N.E.; Taylor, R.A. Boosting solar energy conversion with nanofluids. Phys. Today 2017, 70, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Arefin, M.A. Analysis of an Integrated Photovoltaic Thermal System by Top Surface Natural Circulation of Water. Front. Energy Res. 2019, 7, 97. [Google Scholar] [CrossRef]
- Han, X.; Xue, D.; Zheng, J.; Alelyani, S.M.; Chen, X. Spectral characterization of spectrally selective liquid absorption filters and exploring their effects on concentrator solar cells. Renew. Energy 2019, 131, 938–945. [Google Scholar] [CrossRef]
- Looser, R.; Vivar, M.; Everett, V. Spectral characterisation and long-term performance analysis of various commercial Heat Transfer Fluids (HTF) as Direct-Absorption Filters for CPV-T beam-splitting applications. Appl. Energy 2014, 113, 1496–1511. [Google Scholar] [CrossRef]
- Vivar, M.; Everett, V. A review of optical and thermal transfer fluids used for optical adaptation or beam-splitting in concentrating solar systems. Prog. Photovoltaics Res. Appl. 2014, 22, 612–633. [Google Scholar] [CrossRef]
- Mojiri, A.; Taylor, R.; Thomsen, E.; Rosengarten, G. Spectral beam splitting for efficient conversion of solar energy—A review. Renew. Sustain. Energy Rev. 2013, 28, 654–663. [Google Scholar] [CrossRef]
- Ju, X.; Xu, C.; Han, X.; Du, X.; Wei, G.; Yang, Y. A review of the concentrated photovoltaic/thermal (CPVT) hybrid solar systems based on the spectral beam splitting technology. Appl. Energy 2017, 187, 534–563. [Google Scholar] [CrossRef]
- Othman, M.Y.H.; Hussain, F.; Sopian, K.; Yatim, B.; Ruslan, H. Performance study of air-based photovoltaic-thermal (PV/T) collector with different designs of heat exchanger. Sains Malays. 2013, 42, 1319–1325. [Google Scholar]
- Hjerrild, N.E.; Mesgari, S.; Crisostomo, F.; Scott, J.A.; Amal, R.; Taylor, R.A. Hybrid PV/T enhancement using selectively absorbing Ag–SiO2/carbon nanofluids. Sol. Energy Mater. Sol. Cells 2016, 147, 281–287. [Google Scholar] [CrossRef]
- He, Y.; Hu, Y.; Li, H. An Ag@TiO2/ethylene glycol/water solution as a nanofluid-based beam splitter for photovoltaic/thermal applications in cold regions. Energy Convers. Manag. 2019, 198, 111838. [Google Scholar] [CrossRef]
- Hassani, S.; Saidur, R.; Mekhilef, S.; Taylor, R.A. Environmental and exergy benefit of nanofluid-based hybrid PV/T systems. Energy Convers. Manag. 2016, 123, 431–444. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Kazem, H.A.; Chaichan, M.T.; Sopian, K. Photovoltaic/Thermal (PV/T) Systems: Principles, Design, and Applications; Springer International Publishing: Cham, Switzerland, 2019; ISBN 9783030278243. [Google Scholar]
- Mojiri, A.; Stanley, C.; Rodriguez-Sanchez, D.; Everett, V.; Blakers, A.; Rosengarten, G. A spectral-splitting PV—Thermal volumetric solar receiver. Appl. Energy 2016, 169, 63–71. [Google Scholar] [CrossRef]
- Ni, J.; Li, J.; An, W.; Zhu, T. Performance analysis of nanofluid-based spectral splitting PV/T system in combined heating and power application. Appl. Therm. Eng. 2018, 129, 1160–1170. [Google Scholar] [CrossRef]
- Crisostomo, F.; Hjerrild, N.; Mesgari, S.; Li, Q.; Taylor, R.A. A hybrid PV/T collector using spectrally selective absorbing nanofluids. Appl. Energy 2017, 193, 1–14. [Google Scholar] [CrossRef]
- Abdallah, S.R.; Saidani-Scott, H.; Abdellatif, O.E. Performance analysis for hybrid PV/T system using low concentration MWCNT (water-based) nanofluid. Sol. Energy 2019, 181, 108–115. [Google Scholar] [CrossRef]
- Du, M.; Tang, G.H.; Wang, T.M. Exergy analysis of a hybrid PV/T system based on plasmonic nanofluids and silica aerogel glazing. Sol. Energy 2019, 183, 501–511. [Google Scholar] [CrossRef]
- Shah, T.R.; Ali, H.M. Applications of hybrid nanofluids in solar energy, practical limitations and challenges: A critical review. Sol. Energy 2019, 183, 173–203. [Google Scholar] [CrossRef]
- Han, X.; Chen, X.; Wang, Q.; Alelyani, S.M.; Qu, J. Investigation of CoSO4-based Ag nanofluids as spectral beam splitters for hybrid PV/T applications. Sol. Energy 2019, 177, 387–394. [Google Scholar] [CrossRef]
- Mausam, K.; Kumar, S.; Kumar Ghosh, S.; Kumar Tiwari, A.; Sehgal, M. Solicitation of nanoparticles/fluids in solar thermal energy harvesting: A review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Sahin, A.Z.; Uddin, M.A.; Yilbas, B.S.; Al-Sharafi, A. Performance enhancement of solar energy systems using nanofluids: An updated review. Renew. Energy 2020, 145, 1126–1148. [Google Scholar] [CrossRef]
- Walshe, J.; Amarandei, G.; Ahmed, H.; McCormack, S.; Doran, J. Development of poly-vinyl alcohol stabilized silver nanofluids for solar thermal applications. Sol. Energy Mater. Sol. Cells 2019, 201, 110085. [Google Scholar] [CrossRef]
- Ahmed, A.; Baig, H.; Sundaram, S.; Mallick, T.K. Use of Nanofluids in Solar PV/Thermal Systems. Int. J. Photoenergy 2019, 2019, 8039129. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.S.; Kamnapure, N.R.; Srivastava, S. Nanofluid and nanocomposite applications in solar energy conversion systems for performance enhancement: A review. Int. J. Low-Carbon Technol. 2016, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Dandoutiya, B.K.; Kumar, A. A Review on Nano Fluids for Solar Collector Application. SSRN Electron. J. 2019, 1–10. [Google Scholar] [CrossRef]
- Wahab, A.; Hassan, A.; Qasim, M.A.; Ali, H.M.; Babar, H.; Sajid, M.U. Solar energy systems—Potential of nanofluids. J. Mol. Liq. 2019, 289, 111049. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef]
- Minea, A.A.; Moldoveanu, M.G. Overview of Hybrid Nanofluids Development and Benefits. J. Eng. Thermophys. 2018, 27, 507–514. [Google Scholar] [CrossRef]
- Wole-Osho, I.; Okonkwo, E.C.; Adun, H.; Kavaz, D.; Abbasoglu, S. An intelligent approach to predicting the effect of nanoparticle mixture ratio, concentration and temperature on thermal conductivity of hybrid nanofluids. J. Therm. Anal. Calorim. 2020, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Qian, S.; Zhang, Z. Investigation of the aggregation morphology of nanoparticle on the thermal conductivity of nanofluid by molecular dynamics simulations. Int. J. Heat Mass Transf. 2018, 127, 1138–1146. [Google Scholar] [CrossRef]
- Khalifeh, A.; Vaferi, B. Intelligent assessment of effect of aggregation on thermal conductivity of nanofluids—Comparison by experimental data and empirical correlations. Thermochim. Acta 2019, 681, 178377. [Google Scholar] [CrossRef]
- Tahmooressi, H.; Kasaeian, A.; Tarokh, A.; Rezaei, R.; Hoorfar, M. Numerical simulation of aggregation effect on nanofluids thermal conductivity using the lattice Boltzmann method. Int. Commun. Heat Mass Transf. 2020, 110, 104408. [Google Scholar] [CrossRef]
- Prasher, R.; Evans, W.; Meakin, P.; Fish, J.; Phelan, P.; Keblinski, P. Effect of aggregation on thermal conduction in colloidal nanofluids. Appl. Phys. Lett. 2006, 89, 143119. [Google Scholar] [CrossRef] [Green Version]
- Walshe, J.; Mc Carron, P.; Ahmed, H.; Mc Cormack, S.; Doran, J. Metal Coordination Complexes: A Bottom-Up Approach Tailored Towards Solar Energy Applications BT—Renewable Energy and Sustainable Buildings: Selected Papers from the World Renewable Energy Congress WREC 2018; Sayigh, A., Ed.; Springer International Publishing: Cham, Swizerland, 2020; pp. 719–725. ISBN 978-3-030-18488-9. [Google Scholar]
- Jana, J.; Ganguly, M.; Pal, T. Evolution, Stabilization, and Tuning of Metal-Enhanced Fluorescence in Aqueous Solution. Surf. Plasmon Enhanc. Coupled Control. Fluoresc. 2017, 151–178. [Google Scholar] [CrossRef]
- Eurostat Electricity Price Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Electricity_price_statistics (accessed on 17 May 2020).
- Eurostat Natural Gas Prices. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Natural_gas_price_statistics (accessed on 17 May 2020).
- Gandra, R.M.; Mc Carron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal Potential of Copper(II), Manganese(II) and Silver(I) 1,10-Phenanthroline Chelates Against Multidrug-Resistant Fungal Species Forming the Candida haemulonii Complex: Impact on the Planktonic and Biofilm Lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Gandra, R.M.; McCarron, P.; Viganor, L.; Fernandes, M.F.; Kavanagh, K.; McCann, M.; Branquinha, M.H.; Santos, A.L.S.; Howe, O.; Devereux, M. In vivo Activity of Copper(II), Manganese(II), and Silver(I) 1,10-Phenanthroline Chelates Against Candida haemulonii Using the Galleria mellonella Model. Front. Microbiol. 2020, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- McCarron, P.; McCann, M.; Devereux, M.; Kavanagh, K.; Skerry, C.; Karakousis, P.C.; Aor, A.C.; Mello, T.P.; Santos, A.L.S.; Campos, D.L.; et al. Unprecedented in Vitro Antitubercular Activitiy of Manganese(II) Complexes Containing 1,10-Phenanthroline and Dicarboxylate Ligands: Increased Activity, Superior Selectivity, and Lower Toxicity in Comparison to Their Copper(II) Analogs. Front. Microbiol. 2018, 9, 1432. [Google Scholar] [CrossRef] [PubMed]
- Kellett, A.; O’Connor, M.; McCann, M.; Howe, O.; Casey, A.; McCarron, P.; Kavanagh, K.; McNamara, M.; Kennedy, S.; May, D.D.; et al. Water-soluble bis(1{,}10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: Promising leads for chemotherapeutic drug development. Med. Chem. Commun. 2011, 2, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of dyes in solutions with high absorbance. Inner filter effect correction. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, T.; Morrison, G.; Taylor, R.; Rosengarten, G. Performance of a linear fresnel rooftop mounted concentrating solar collector. In Proceedings of the 50th Australian Solar Energy Society Annual Conference (AuSE), Melbourne, Australia, 6–7 December 2012; AuSE: Melbourne, Australia, 2012; pp. 6–7. [Google Scholar]
- Han, X.; Chen, X.; Sun, Y.; Qu, J. Performance improvement of a PV/T system utilizing Ag/CoSO4-propylene glycol nanofluid optical filter. Energy 2020, 192, 116611. [Google Scholar] [CrossRef]
- Sarkar, M.N.I. Effect of various model parameters on solar photovoltaic cell simulation: A SPICE analysis. Renew. Wind. Water Sol. 2016, 3, 13. [Google Scholar] [CrossRef]
- GlobalPetrolPrices Electricity Prices in China. Available online: https://www.globalpetrolprices.com/China/electricity_prices/ (accessed on 17 May 2020).
- GlobalPetrolPrices Canda Electricty Prices. Available online: https://www.globalpetrolprices.com/Canada/electricity_prices/ (accessed on 17 May 2020).
- CEIC China Gas Price. Available online: https://www.ceicdata.com/en/china/gas-price (accessed on 17 May 2020).
- Oxford Martin School Natural Gas Prices: 2009 to 2018. Available online: https://ourworldindata.org/grapher/natural-gas-prices (accessed on 17 May 2020).





| Abbreviation | Chemical Formula | Name | Molecular Weight (g mol−1) |
|---|---|---|---|
| P183 | C32H18N8 | 1,4-bis(1H-imidazo[4,5-f][1,10]phenanthroline-2-yl)benzene | 514.55 |
| P205 | C20H11N5 | 4-(1H-imidazo[4,5-f][1,10]phenanthroline-2-yl)benzonitrile | 321.44 |
| P282 | C29H16N4 | 2-(pyren-1-yl)-1H-imidazo[4,5-f][1,10]phenanthroline | 420.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walshe, J.; Carron, P.M.; McLoughlin, C.; McCormack, S.; Doran, J.; Amarandei, G. Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications. Nanomaterials 2020, 10, 1201. https://doi.org/10.3390/nano10061201
Walshe J, Carron PM, McLoughlin C, McCormack S, Doran J, Amarandei G. Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications. Nanomaterials. 2020; 10(6):1201. https://doi.org/10.3390/nano10061201
Chicago/Turabian StyleWalshe, James, Pauraic Mc Carron, Conor McLoughlin, Sarah McCormack, John Doran, and George Amarandei. 2020. "Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications" Nanomaterials 10, no. 6: 1201. https://doi.org/10.3390/nano10061201
APA StyleWalshe, J., Carron, P. M., McLoughlin, C., McCormack, S., Doran, J., & Amarandei, G. (2020). Nanofluid Development Using Silver Nanoparticles and Organic-Luminescent Molecules for Solar-Thermal and Hybrid Photovoltaic-Thermal Applications. Nanomaterials, 10(6), 1201. https://doi.org/10.3390/nano10061201