Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Silica Nanoparticles Preparation
2.2. Samples Characterization
3. Results and Discussion
3.1. Size, Cobalt Content, and Spectral Properties of the Hybrid Nanoparticles (CoII@SiO2) in Correlation with the Synthetic Conditions
3.2. Nanoarchitecture of CoII@SiO2 and SiO2 in Correlation with the Synthetic Procedure
3.3. Electrochemical Behavior of CoII@SiO2
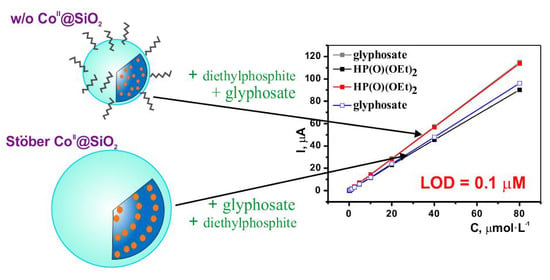
3.4. CoII@SiO2 for Electrochemical Determination of OPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antony, R.; Manickam, S.T.D.; Balakumar, S. Cu(II), Co(II) and Ni(II) Complexes Installed on Functionalized Silica Surface for Hydrogen Peroxide Assisted Cyclohexane Oxidation. J. Inorg. Organomet. Polym. Mater. 2017, 27, 418–426. [Google Scholar] [CrossRef]
- Vashurin, A.; Marfin, Y.; Tarasyuk, I.; Kuzmin, I.; Znoyko, S.; Goncharenko, A.; Rumyantsev, E. Sulfonated octa-substituted Co(II) phthalocyanines immobilized on silica matrix as catalyst for Thiuram E synthesis. Appl. Organomet. Chem. 2018, 32, e4482. [Google Scholar] [CrossRef]
- Faraji, A.R.; Mosazadeh, S.; Ashouri, F. Synthesis and characterization of cobalt-supported catalysts on modified magnetic nanoparticle: Green and highly efficient heterogeneous nanocatalyst for selective oxidation of ethylbenzene, cyclohexene and oximes with molecular oxygen. J. Colloid Interface Sci. 2017, 506, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Luque, R.; Clark, J.H.; Karimi, B.; Macquarrie, D.J. A silica supported cobalt (II) Salen complex as efficient and reusable catalyst for the selective aerobic oxidation of ethyl benzene derivatives. Catal. Commun. 2011, 12, 510–513. [Google Scholar] [CrossRef]
- Chen, L.; Li, B.-D.; Xu, Q.-X.; Liu, D.-B. A silica gel supported cobalt(II) Schiff base complex as efficient and recyclable heterogeneous catalyst for the selective aerobic oxidation of alkyl aromatics. Chin. Chem. Lett. 2013, 24, 849–852. [Google Scholar] [CrossRef]
- Islam, S.M.; Ghosh, K.; Molla, R.A.; Roy, A.S.; Salam, N.; Iqubal, M.A. Synthesis of a reusable polymer anchored cobalt(II) complex for the aerobic oxidation of alkyl aromatics and unsaturated organic compounds. J. Organomet. Chem. 2014, 774, 61–69. [Google Scholar] [CrossRef]
- Marfin, Y.S.; Vashurin, A.S.; Rumyantsev, E.V.; Puhovskaya, S.G. Sol–gel synthesis of highly effective catalyst based on cobalt tetrasulfophthalocyanine complex and silicon oxide. J. Sol Gel Sci. Technol. 2013, 66, 306–311. [Google Scholar] [CrossRef]
- Ranaweera, S.A.; Rowe, M.D.; Walters, K.B.; Henry, W.P.; White, M.G.; Rodriguez, J.M. Synthesis, characterization and catalytic activity of a cobalt catalyst: Silica-supported, bis(1,5-diphenyl-1,3,5-pentanetrionato)dicobalt(II)[Co2(dba)2]. Appl. Catal. A Gen. 2017, 529, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Li-Juan, C.; Fu-Ming, M.; Guang-Xing, L. Co(II) Schiff base complexes on silica by sol–gel method as heterogeneous catalysts for oxidative carbonylation of aniline. Catal. Commun. 2009, 10, 981–985. [Google Scholar] [CrossRef]
- Trujillano, R.; Lambert, J.-F.; Louis, C. Chemistry of Silica-Supported Cobalt Catalysts Prepared by Cation Adsorption. 2. Neoformation of Cobalt Phyllosilicate. J. Phys. Chem. C 2008, 112, 18551–18558. [Google Scholar] [CrossRef]
- Yakubovich, T.N.; Ermokhina, N.I.; Bratushko, Y.I.; Zub, Y.L.; Chuiko, A.A. Cobalt complex with 2.2′-bipyridyl immobilized on disperse silica surface in the reaction of p-hydroquinone oxidation. Comparison of the homogeneous and heterogeneous catalysis results. In Dioxygen Activation and Homogeneous Catalytic Oxidation; Simindi, L.I., Ed.; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1991; pp. 179–188. [Google Scholar]
- Rautiainen, A.; Lindblad, M.; Backman, L.B.; Puurunen, R.L. Preparation of silica-supported cobalt catalysts through chemisorption of cobalt(II) and cobalt(III) acetylacetonate. Phys. Chem. Chem. Phys. 2002, 4, 2466–2472. [Google Scholar] [CrossRef]
- Yuanfa, Y.; Renxian, Z.; Shaofen, Z.; Qiaoling, L.; Xiaoming, Z. Silica-supported poly-γ-aminopropylsilane Ni2+, Cu2+, Co2+ complexes: Efficient catalysts for Heck vinylation reaction. J. Mol. Catal. A Chem. 2003, 192, 303–306. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Fedorenko, S.V.; Strekalova, S.O.; Kholin, K.V.; Mustafina, A.R.; Zhilkin, M.Y.; Khrizanforova, V.V.; Osin, Y.N.; Salnikov, V.V.; Gryaznova, T.V.; et al. A Ni(III) complex stabilized by silica nanoparticles as an efficient nanoheterogeneous catalyst for oxidative C–H fluoroalkylation. Dalton Trans. 2016, 45, 11976–11982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khrizanforov, M.N.; Fedorenko, S.V.; Mustafina, A.R.; Khrizanforova, V.V.; Kholin, K.V.; Nizameev, I.R.; Gryaznova, T.V.; Grinenko, V.V.; Budnikova, Y.H. Nano-architecture of silica nanoparticles as a tool to tune both electrochemical and catalytic behavior of NiII@SiO2. RSC Adv. 2019, 9, 22627–22635. [Google Scholar] [CrossRef] [Green Version]
- Budnikova, Y.; Bochkova, O.; Khrizanforov, M.; Nizameev, I.; Kholin, K.; Gryaznova, T.; Laskin, A.; Dudkina, Y.; Strekalova, S.; Fedorenko, S.; et al. Selective C(sp2)-H Amination Catalyzed by High-Valent Cobalt(III)/(IV)-bpy Complex Immobilized on Silica Nanoparticles. ChemCatChem 2019, 11, 5615–5624. [Google Scholar] [CrossRef]
- Ryabchuk, P.; Agostini, G.; Pohl, M.-M.; Lund, H.; Agapova, A.; Junge, H.; Junge, K.; Beller, M. Intermetallic nickel silicide nanocatalyst-A non-noble metal–based general hydrogenation catalyst. Sci. Adv. 2018, 4, eaat0761. [Google Scholar] [CrossRef] [Green Version]
- Dudkina, Y.B.; Gryaznova, T.V.; Osin, Y.N.; Salnikov, V.V.; Davydov, N.A.; Fedorenko, S.V.; Mustafina, A.R.; Vicic, D.A.; Sinyashin, O.G.; Budnikova, Y.H. Nanoheterogeneous catalysis in electrochemically induced olefin perfluoroalkylation. Dalton Trans. 2015, 44, 8833–8838. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Karimi-Maleh, H.; Tahernejad-Javazmi, F. Fabrication of an electroanalytical sensor for determination of deoxyepinephrine in the presence of uric acid using CuFe2O4 nanoparticle/ionic liquid amplified sensor. J. Electrochem. Soc. 2019, 166, H218. [Google Scholar] [CrossRef]
- Liu, X. Electrochemical sensor for determination of parathion based on electropolymerization poly (safranine) film electrode. Int. J. Electrochem. 2011, 2011, 986494. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Luona, W.; Chengyin, W.; Xiaoya, H.; Yunling, L.; Guoxiu, W. Sensitive detection of glyphosate based on a Cu-BTC MOF/g-C3N4 nanosheet photoelectrochemical sensor. Electrochim. Acta 2019, 317, 341–347. [Google Scholar] [CrossRef]
- Shelkovnikov, V.V.; Novolokov, K.Y. Voltammetric sensor for determining malathion and diazinon. Anal. Control 2019, 23, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Tadayon, F.; Jahromi, M.N. A sensitive and selective electrochemical determination of Diazinon based on Au–Pd/rGO–MWCNTs composite: Analytical application in water, fruit and vegetable samples. J. Iran. Chem. Soc. 2020, 17, 847–857. [Google Scholar] [CrossRef]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zahirifar, F.; Rahimnejad, M.; Abdulkareem, R.A.; Najafpour, G. Determination of Diazinon in fruit samples using electrochemical sensor based on carbon nanotubes modified carbon paste electrode. Biocatal. Agric. Biotechnol. 2019, 20, 101245. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modified metal-organic framework sensing platforms. Bioelectrochemistry 2019, 130, 107348. [Google Scholar] [CrossRef] [PubMed]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef]
- Hua, Q.T.; Ruecha, N.; Hiruta, Y.; Citterio, D. Disposable electrochemical biosensor based on surface-modified screen-printed electrodes for organophosphorus pesticide analysis. Anal. Methods 2019, 11, 3439–3445. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, Q.; Dong, H.; Li, F.; Zhang, Y.; Guo, Y.; Sun, X. Acetylcholinesterase biosensor modified with ATO/OMC for detecting organophosphorus Pesticides. New J. Chem. 2019, 43, 946–952. [Google Scholar] [CrossRef]
- Tefera, M.; Admassie, S.; Tessema, M.; Mehretie, S. Electrochemical sensor for determination of fenitrothion at multi-wall carbon nanotubes modified glassy carbon electrode. Anal. Bioanal. Chem. Res. 2015, 2, 139–150. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Evtyugin, G.A.; Budnikova, Y.G.; Al′fonsov, V.A. Chemically modified electrodes with amperometric response in enantioselective analysis. J. Anal. Chem. 2008, 63, 2–12. [Google Scholar] [CrossRef]
- Khanmohammadi, A.; Ghazizadeh, A.J.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An overview to electrochemical biosensors and sensors for the detection of environmental contaminants. J. Iran. Chem. Soc. 2020, 1–19. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A. Recent advances in voltammetric, amperometric and ion-selective (bio)sensors fabricated by microengineering manufacturing approaches. Curr. Opin. Electrochem. 2020, 23, 21–25. [Google Scholar] [CrossRef]
- Wu., L.; Yan, H.; Wang, J.; Liu, G.; Xie, W. Tyrosinase Incorporated with Au-Pt@SiO2 Nanospheres for Electrochemical Detection of Bisphenol, A. J. Electrochem. Soc. 2019, 166, B562. [Google Scholar] [CrossRef]
- Jeerapan, I.; Poorahong, S. Review-Flexible and Stretchable Electrochemical Sensing Systems: Materials, Energy Sources, and Integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Patel, B.R.; Noroozifar, M.; Kerman, K. Review—Nanocomposite-Based Sensors for Voltammetric Detection of Hazardous Phenolic Pollutants in Water. J. Electrochem. Soc. 2020, 167, 037568. [Google Scholar] [CrossRef]
- Zhu, N.; Cai, H.; He, P.; Fang, Y. Tris(2,2′-bipyridyl)cobalt(III)-doped silica nanoparticle DNA probe for the electrochemical detection of DNA hybridization. Anal. Chim. Acta 2003, 481, 181–189. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Xu, J.; Liang, J.; Li, J.; Yang, W. Tuning the Emission Properties of Ru(phen)32+ Doped Silica Nanoparticles by Changing the Addition Time of the Dye during the Stöber Process. Langmuir 2010, 26, 6657–6662. [Google Scholar] [CrossRef]
- Lian, W.; Litherland, S.A.; Badrane, H.; Tan, W.; Wu, D.; Baker, H.V.; Gulig, P.A.; Lim, D.V.; Jin, S. Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal. Biochem. 2004, 334, 135–144. [Google Scholar] [CrossRef]
- Ahkam, Q.M.; Khan, E.U.; Iqbal, J.; Murtaza, A.; Khan, M.T. Synthesis and characterization of cobalt-doped SiO2 nanoparticles. Phys. B Condens. Matter 2019, 572, 161–167. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Env. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Gryaznova, T.; Sinyashin, O.; Budnikova, Y. Aromatic perfluoroalkylation with metal complexes in electrocatalytic conditions. J. Organomet. Chem. 2012, 718, 101–104. [Google Scholar] [CrossRef]
- Mustafina, A.R.; Fedorenko, S.V.; Konovalova, O.D.; Menshikova, A.Y.; Shevchenko, N.N.; Soloveva, S.E.; Konovalov, A.I.; Antipin, I.S. Novel Highly Charged Silica-Coated Tb(III) Nanoparticles with Fluorescent Properties Sensitive to Ion Exchange and Energy Transfer Processes in Aqueous Dispersions. Langmuir 2009, 25, 3146–3151. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.P.; Gerasimova, T.P.; Ermolaev, V.V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Sinyashin, O.G.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- DIFFRAC Plus Evaluation package EVA; Version 11; User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005.
- Small Angle X-ray Scattering; Version 4.0; Software Reference Manual, M86-E00005-0600; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.; Koch, M.H.J.; Svergun, D.I. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- Arriagada, F.J.; Osseo-Asare, K. Controlled hydrolysis of tetraethoxysilane in a nonionic water-in-oil microemulsion: A statistical model of silica nucleation. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 311–326. [Google Scholar] [CrossRef]
- Liver, E. Elektronnaya Spektroskopiya Neorganicheskih Soedineniy (Electron Spectroscopy of Inorganic Compounds), 2nd ed.; Mir: Moscow, Russia, 1987. [Google Scholar]
- Alexandru, M.-G.; Velickovic, M.; Hrubaru, K.M.-M.; Draghici, C. Two complexes of Co(II) and Pd(II) formed in reaction with a mono-T.C. oxazoline derivative. Spectroscopic characterization and cytotoxic evaluation. J. Mol. Struct. 2013, 1041, 55–60. [Google Scholar] [CrossRef]
- Verberckmoes, A.A.; Weckhuysen, B.M.; Schoonheydt, R.A. Spectroscopy and coordination chemistry of cobalt in molecular sieves. Microporous Mesoporous Mater. 1998, 22, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Gerasimova, T.; Shekurov, R.; Gilmanova, L.; Laskin, A.; Katsyuba, S.; Kovalenko, V.; Khrizanforov, M.; Milyukov, V.; Sinyashin, O. IR and UV study of reversible water-induced structural transformations of poly(manganese 1,10 -ferrocenediyl-bis(Hphosphinate)) and poly(cobalt 1,10-ferrocenediyl-bis(H-phosphinate)). J. Mol. Struct. 2018, 1166, 237–242. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforova, V.; Gilmanova, L.; Khrizanforov, M.; Miluykov, V.; Kataeva, O.; Yamaleeva, Z.; Burganov, T.; Gerasimova, T.; Khamatgalimov, A.; et al. Zn and Co redox active coordination polymers as efficient electrocatalysts. Dalton Trans. 2019, 48, 3601–3609. [Google Scholar] [CrossRef]
- Yousef, T.A.; El-Reash, G.M.A.; El-Gammal, O.A.; Bedier, R.A. Co(II), Cu(II), Cd(II), Fe(III) and U(VI) complexes containing a NSNO donor ligand: Synthesis, characterization, optical band gap, in vitro antimicrobial and DNA cleavage studies. J. Mol. Struct. 2012, 1029, 149–160. [Google Scholar] [CrossRef]
- Kang, W.; Spanjers, C.S.; Rioux, R.M.; Hoefelmeyer, J.D. Synthesis of brookite TiO2nanorods with isolated Co(II) surface sites and photocatalytic degradation of 5,8-dihydroxy-1,4-naphthoquinone dye. J. Mater. Chem. A 2013, 1, 7717–7728. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Aziz, M.S.B.A.; Duralima, M.B.; Riaz, S.; Naseemb, S.; Abdullha, M.B.; Osman, S.S. CR incorporation in mesoporous silica matrix for fiber optic pH sensing. Sens. Actuators A Phys. 2018, 280, 429–436. [Google Scholar] [CrossRef]
- Islam, S.; Bakhtiar, H.; Naseem, S.; Aziz, M.S.B.A.; Biden, N.; Riaz, S.; Ali, J. Surface functionality and optical properties impact of phenol red dye on mesoporous silica matrix for fiber optic pH sensing. Sens. Actuators A Phys. 2018, 276, 267–277. [Google Scholar] [CrossRef]
- Chen, C.; Xu, J.; Zhang, Q.; Ma, H.; Miao, H.; Zhou, L. Direct synthesis of bifunctionalized hexagonal mesoporous silicas and its catalytic performance for aerobic oxidation of cyclohexane. J. Phys. Chem. C 2009, 113, 2855–2860. [Google Scholar] [CrossRef]
- Fouad, O.A.; Makhlouf, S.A.; Ali, G.A.M.; El-Sayed, A.Y. Cobalt/silica nanocomposite via thermal calcination-reduction of gel precursors. Mater. Chem. Phys. 2011, 128, 70–76. [Google Scholar] [CrossRef]
- Genovese, D.; Rampazzo, E.; Bonacchi, S.; Montalti, M.; Zaccheroni, N.; Prodi, L. Energy transfer processes in dye-doped nanostructures yield cooperative and versatile fluorescent probes. Nanoscale 2014, 6, 3022–3036. [Google Scholar] [CrossRef] [PubMed]
- Koirala, R.; Safonova, O.V.; Pratsinis, S.E.; Baiker, A. Effect of cobalt loading on structure and catalytic behavior of CoOx/SiO2 in CO2-assisted dehydrogenation of ethane. Appl. Catal. A 2018, 552, 77–85. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nagata, K.; Adachi, T.; Imanaka, T.; Inamura, K.; Takyu, T. Preparation and Characterization of Highly Dispersed Cobalt Oxide and Sulfide Catalysts Supported on SiO2. J. Phys. Chem. 1991, 95, 310–319. [Google Scholar] [CrossRef]
- Masalov, V.M.; Kudrenko, E.A.; Grigoryeva, N.A.; Ezdakova, K.V.; Roddatis, V.V.; Sukhinina, N.S.; Arefev, M.V.; Mistonov, A.A.; Grigoriev, S.V.; Emelchenko, G.A. Direct observation of the shell-like structure of SiO2 partices synthesized by the multistage Stober method. Nano Brief Rep. Rev. 2013, 8, 1350036. [Google Scholar] [CrossRef] [Green Version]
- Fouilloux, S.; Désert, A.; Taché, O.; Spalla, O.; Daillant, J.; Thill, A. SAXS exploration of the synthesis of ultra monodisperse silica nanoparticles and quantitative nucleation growth modeling. J. Colloid Interface Sci. 2010, 346, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fouilloux, S.; Daillant, J.; Thill, A. Single step synthesis of 5–30 nm monodisperse silica nanoparticles: Important experimental parameters and modeling. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 122–127. [Google Scholar] [CrossRef]
- De Almeida, L.K.S.; Chigome, S.; Torto, N.; Frost, C.L.; Pletschke, B.I. A novel colorimetric sensor strip for the detection of glyphosate in water. Sens. Actuators B Chem. 2015, 206, 357–363. [Google Scholar] [CrossRef]










| Elements | C | H | B | Co | F | N |
|---|---|---|---|---|---|---|
| Anal. Calc., % | 51.39 | 3.45 | 3.08 | 8.41 | 21.68 | 11.99 |
| Found, % | 51.41 | 3.41 |
| Synthetic Method, Precursor | d, nm | Si:Co Molar Ratio, % | D, nm | PDI | ζ, mV |
|---|---|---|---|---|---|
| Stöber, CoII(bpy)3 | 109 ± 20 | 100:0.243 | 225 ± 2 | 0.139 | −51 ± 1 |
| w/o, CoII(bpy)3 | 50 ± 5 1 | 100:0.680 | 144 ± 1 | 0.114 | −29 ± 1 |
| Stöber, CoCl2 | 118 ± 10 | 100:0.130 | 154 ± 1 | 0.144 | −51 ± 1 |
| w/o, CoCl2 | 43 ± 3 | 100:0.578 | 118± 2 | 0.117 | −38 ± 1 |
| Sample (Technique, Precursor) | Rg, Å | Rg*, Å | Dmax, nm | Vpart, Å3 | I0, a.u. | ds, nm | FD | dTEM, nm |
|---|---|---|---|---|---|---|---|---|
| w/o, CoCl2 | 131.0 ± 6 | 130.9 ± 7 | 39.4 ± 0.6 | 122 × 105 | 166000 | 33.8 ± 1.8 | 3.41 | 43 ± 3 |
| w/o, CoII(bpy)3 | 145.5 ± 7 | 145.6 ± 5 | 43.4 ± 0.7 | 148 × 105 | 229900 | 37.6 ± 1.3 | 3.29 | 50 ± 5 |
| Stöber, CoCl2 | 164.6 ± 7 | 164.4 ± 8 | 50.1 ± 0.7 | 236 × 105 | 84660 | 41.4 ± 2.1 | 2.61 | 118 ± 10 |
| Stöber, CoII(bpy)3 | 139.4 ± 6 | 139.4 ± 7 | 43.7 ± 0.6 | 133 × 105 | 60640 | 36.0 ± 1.8 | 2.69 | 109 ± 20 |
| w/o, “empty” | 128.0 ± 6 | 127.8 ± 7 | 36.4 ± 0.6 | 114 × 105 | 183600 | 33.0 ± 1.8 | 3.53 | 48 ± 5 |
| Stöber, “empty” | 132.7 ± 8 | 132.6 ± 6 | 37.1 ± 0.6 | 101 × 105 | 143200 | 34.0 ± 1.5 | 3.37 | 97 ± 5 |
| Sample | Reduction | Oxidation | |
|---|---|---|---|
| 1Epc/Epa, V | 2Epc/Epa, V | 1Epa/Epc, V | |
| Stöber CoII@SiO2 | −1.12/−0.90 | −1.69/irrev | 0.71/irrev |
| w/o CoII@SiO2 | −1.15/−0.99 | −1.38/−1.26 | 0.72/irrev |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochkova, O.; Khrizanforov, M.; Gubaidullin, A.; Gerasimova, T.; Nizameev, I.; Kholin, K.; Laskin, A.; Budnikova, Y.; Sinyashin, O.; Mustafina, A. Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials 2020, 10, 1338. https://doi.org/10.3390/nano10071338
Bochkova O, Khrizanforov M, Gubaidullin A, Gerasimova T, Nizameev I, Kholin K, Laskin A, Budnikova Y, Sinyashin O, Mustafina A. Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials. 2020; 10(7):1338. https://doi.org/10.3390/nano10071338
Chicago/Turabian StyleBochkova, Olga, Mikhail Khrizanforov, Aidar Gubaidullin, Tatiana Gerasimova, Irek Nizameev, Kirill Kholin, Artem Laskin, Yulia Budnikova, Oleg Sinyashin, and Asiya Mustafina. 2020. "Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability" Nanomaterials 10, no. 7: 1338. https://doi.org/10.3390/nano10071338
APA StyleBochkova, O., Khrizanforov, M., Gubaidullin, A., Gerasimova, T., Nizameev, I., Kholin, K., Laskin, A., Budnikova, Y., Sinyashin, O., & Mustafina, A. (2020). Synthetic Tuning of CoII-Doped Silica Nanoarchitecture Towards Electrochemical Sensing Ability. Nanomaterials, 10(7), 1338. https://doi.org/10.3390/nano10071338