Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Analysis
3.2. Morphological Analysis
3.3. FTIR Spectra Analysis
3.4. Physical Analysis of the Film Heaters
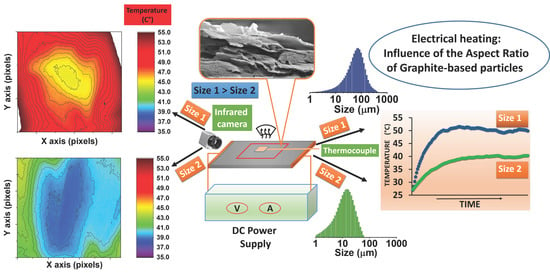
3.5. Electrical Heating Behavior (Constant Current)
3.6. Electrical Heating Behavior (Constant Voltage)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shinkafi, A.; Lawson, C. Enhanced method of conceptual sizing of aircraft electro-thermal de-icing system. Int. J. Mech. Aerosp. Ind. Mech. Eng. 2014, 8, 1069–1076. [Google Scholar]
- Arianpour, F.; Farzaneh, M.; Kulinich, S. Hydrophobic and ice-retarding properties of doped silicone rubber coatings. Appl. Surf. Sci. 2013, 265, 546–552. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Liu, Y.; Gou, Y.; Wang, L. Effect of contact angle on water droplet freezing process on a cold flat surface. Exp. Therm. Fluid Sci. 2012, 40, 74–80. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Mazzola, L.; Memon, H.; Barman, T.; Turnbull, B.; Mingione, G.; Choi, K.-S.; Hou, X. Development and evaluation of poly (dimethylsiloxane) based composite coatings for icephobic applications. Surf. Coat. Technol. 2018, 349, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhao, Y.; Lv, R.; Yang, C. Freezing of sessile water droplet for various contact angles. Int. J. Therm. Sci. 2016, 101, 59–67. [Google Scholar] [CrossRef]
- Leite, F.L.; Bueno, C.C.; Da Róz, A.L.; Ziemath, E.C.; Oliveira, O.N. Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy. Int. J. Mol. Sci. 2012, 13, 12773–12856. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Young, T. Third. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 65–87. [Google Scholar]
- Li, J.; Zhao, Y.; Hu, J.; Shu, L.; Shi, X. Anti-icing performance of a superhydrophobic PDMS/modified nano-silica hybrid coating for insulators. J. Adhes. Sci. Technol. 2012, 26, 665–679. [Google Scholar] [CrossRef]
- Meier, O.; Scholz, D. A Handbook Method for the Estimation of Power Requirements for Electrical de-Icing Systems; DLRK: Hamburg, Germany, 2010; p. 31. [Google Scholar]
- Alemour, B.; Badran, O.; Hassan, M.R. A Review of Using Conductive Composite Materials in Solving Lightening Strike and Ice Accumulation Problems in Aviation. J. Aerosp. Technol. Manag. 2019, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/carbon nanotube nano composite fibers—A review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef] [PubMed]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci 2010, 35, 357–401. [Google Scholar]
- Chandrasekaran, S.; Seidel, C.; Schulte, K. Preparation and characterization of graphite nano-platelet (GNP)/epoxy nano-composite: Mechanical, electrical and thermal properties. Eur. Polym. J. 2013, 49, 3878–3888. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Saravanan, N.; Rajasekar, R.; Mahalakshmi, S.; Sathishkumar, T.; Sasikumar, K.; Sahoo, S. Graphene and modified graphene-based polymer nanocomposites—A review. J. Reinf. Plast. Compos. 2014, 33, 1158–1170. [Google Scholar] [CrossRef]
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing polymer composite materials: Carbon nanotubes or graphene? Adv. Mater. 2013, 25, 5153–5176. [Google Scholar] [CrossRef] [PubMed]
- Volman, V.; Zhu, Y.; Raji, A.-R.O.; Genorio, B.; Lu, W.; Xiang, C.; Kittrell, C.; Tour, J.M. Radio-frequency-transparent, electrically conductive graphene nanoribbon thin films as deicing heating layers. ACS Appl. Mater. Interfaces 2014, 6, 298–304. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Russo, S.; Lafdi, K.; Tucci, V.; Spinelli, G.; Lamberti, P. Influence of carbon nanoparticles/epoxy matrix interaction on mechanical, electrical and transport properties of structural advanced materials. Nanotechnology 2017, 28, 094001. [Google Scholar] [CrossRef]
- Guadagno, L.; Naddeo, C.; Raimondo, M.; Barra, G.; Vertuccio, L.; Sorrentino, A.; Binder, W.H.; Kadlec, M. Development of self-healing multifunctional materials. Compos. B Eng. 2017, 128, 30–38. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Mauro, M.; Guerra, G.; Lafdi, K.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Optimization of graphene-based materials outperforming host epoxy matrices. RSC Adv. 2015, 5, 36969–36978. [Google Scholar] [CrossRef] [Green Version]
- Bekyarova, E.; Thostenson, E.; Yu, A.; Kim, H.; Gao, J.; Tang, J.; Hahn, H.; Chou, T.-W.; Itkis, M.; Haddon, R. Multiscale carbon nanotube—Carbon fiber reinforcement for advanced epoxy composites. Langmuir 2007, 23, 3970–3974. [Google Scholar] [CrossRef] [PubMed]
- Kandare, E.; Khatibi, A.A.; Yoo, S.; Wang, R.; Ma, J.; Olivier, P.; Gleizes, N.; Wang, C.H. Improving the through-thickness thermal and electrical conductivity of carbon fibre/epoxy laminates by exploiting synergy between graphene and silver nano-inclusions. Compos. Part. A Appl. Sci. Manuf. 2015, 69, 72–82. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, Z.; Bilotti, E.; Peijs, T. Graphite nanoplatelet modified epoxy resin for carbon fibre reinforced plastics with enhanced properties. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase. Compos. B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Naddeo, C.; Raimondo, M.; Barra, G.; De Nicola, F.; Volponi, R.; Lamberti, P.; Spinelli, G.; Tucci, V. Electrical Current Map and Bulk Conductivity of Carbon Fiber-Reinforced Nanocomposites. Polymers 2019, 11, 1865. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, L.; Vietri, U.; Raimondo, M.; Vertuccio, L.; Barra, G.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V.; De Nicola, F. Correlation between electrical conductivity and manufacturing processes of nanofilled carbon fiber reinforced composites. Compos. B Eng. 2015, 80, 7–14. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Naddeo, C.; Barra, G.; Longo, P.; Lamberti, P.; Spinelli, G.; Nobile, M. Morphological, rheological and electrical properties of composites filled with carbon nanotubes functionalized with 1-pyrenebutyric acid. Compos. B Eng. 2018, 147, 12–21. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Fragiadakis, D.; Pissis, P. Reinforcement effect of carbon nanofillers in an epoxy resin system: Rheology, molecular dynamics, and mechanical studies. J. Polym. Sci. Pol. Phys. 2005, 43, 522–533. [Google Scholar] [CrossRef]
- Nobile, M.R.; Fierro, A.; Rosolia, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Viscoelastic properties of graphene-based epoxy resins. In Proceedings of the AIP Conference Proceedings, Salerno, Italy, 15–17 October 2015; p. 020050. [Google Scholar]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457. [Google Scholar] [CrossRef] [PubMed]
- Šimek, P.; Sofer, Z.; Jankovský, O.; Sedmidubský, D.; Pumera, M. Oxygen-Free Highly Conductive Graphene Papers. Adv. Funct. Mater. 2014, 24, 4878–4885. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Vertuccio, L.; De Santis, F.; Pantani, R.; Lafdi, K.; Guadagno, L. Effective de-icing skin using graphene-based flexible heater. Compos. B Eng. 2019, 162, 600–610. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y. Studies on the Electro-Impulse De-Icing System of Aircraft. Aerospace 2019, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Hermans, P.H.; Weidinger, A. On the determination of the crystalline fraction of polyethylenes from X-ray diffraction. Die Makromol. Chem. Macromol. Chem. Phys. 1961, 44, 24–36. [Google Scholar] [CrossRef]
- Tadokoro, H. Structure of Crystalline Polymers; Krieger Pub Co.: Malabar, FL, USA, 1979. [Google Scholar]
- Ju, H.-M.; Huh, S.H.; Choi, S.-H.; Lee, H.-L. Structures of thermally and chemically reduced graphene. Mater. Lett. 2010, 64, 357–360. [Google Scholar] [CrossRef]
- Ferrari, A.; Rodil, S.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef] [Green Version]
- Vidano, R.; Fischbach, D.; Willis, L.; Loehr, T. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Shin, Y.-R.; Sohn, G.-J.; Choi, H.-J.; Bae, S.-Y.; Mahmood, J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Chang, D.W. Edge-carboxylated graphene nanosheets via ball milling. Proc. Natl. Acad. Sci. USA 2012, 109, 5588–5593. [Google Scholar] [CrossRef] [Green Version]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.-S.; Lee, J.H. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Sridhar, V.; Oh, I.-K. A coagulation technique for purification of graphene sheets with graphene–reinforced PVA hydrogel as byproduct. J. Colloid Interface Sci. 2010, 348, 384–387. [Google Scholar] [CrossRef]
- Debelak, B.; Lafdi, K. Use of exfoliated graphite filler to enhance polymer physical properties. Carbon 2007, 45, 1727–1734. [Google Scholar] [CrossRef]
- Emadi, K.; Ehsani, M. Aircraft power systems: Technology, state of the art, and future trends. IEEE Aerosp. Electron. Syst. 2000, 15, 28–32. [Google Scholar] [CrossRef]
- Nya, B.; Brombach, J.; Schulz, D. Benefits of higher voltage levels in aircraft electrical power systems. In Proceedings of the 2012 Electrical Systems for Aircraft, Railway and Ship Propulsion, Bologna, Italy, 16–18 October 2012; pp. 1–5. [Google Scholar]
- Wheeler, P.W.; Clare, J.C.; Trentin, A.; Bozhko, S. An overview of the more electrical aircraft. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2013, 227, 578–585. [Google Scholar] [CrossRef]
- Rutherford, R.B. De-ice and Anti-ice System and Method for Aircraft Surfaces. Google Patents US6194685B1, 27 February 2001. [Google Scholar]
- Yan, J.; Jeong, Y.G. Highly elastic and transparent multiwalled carbon nanotube/polydimethylsiloxane bilayer films as electric heating materials. Mater. Des. 2015, 86, 72–79. [Google Scholar] [CrossRef]
- Redondo, O.; Prolongo, S.; Campo, M.; Sbarufatti, C.; Giglio, M. Anti-icing and de-icing coatings based Joule’s heating of graphene nanoplatelets. Compos. Sci. Technol. 2018, 164, 65–73. [Google Scholar] [CrossRef]










| Method | Device | Characterized Sample | Procedure Measurement/ Technical Specifications |
|---|---|---|---|
| Wide-angle X-ray diffraction (WAXD) | Bruker D8 Advance diffractometer (Bruker, Billerica, MA, USA) | Fillers | According to ref [36] |
| Scanning Electron Microscopy (SEM) | JSM-6700F, (JEOL Akishima, Tokyo, Japan) | Fillers | According to ref [36] |
| Transmission Electron Microscopy (TEM) | JEOL model JEM-1400 Plus (JEOL Akishima, Tokyo, Japan) | Fillers | Resolution image 0.38 nm between dots and 0.2 nm between lines. |
| Raman Spectroscopy | Renishaw inVia (Renishaw Wotton-under-Edge, U.K.) | Fillers | According to ref [36] |
| FTIR spectroscopy | BRUKER Vertex70 (Bruker, Billerica, Ma, USA) | Fillers/film | According to ref [36] |
| Dynamic mechanical analysis (DMA) | Tritec 2000 DMA-Triton Technology (Triton Technology, Grantham, UK) | Film (0.3 × 10 × 15 mm3) | Three points bending mode Amplitude = 0.05 mm; According to ref [36] |
| Differential Scanning Calorimetry (DSC) | Mettler DSC 822/400 (Mettler Toledo) (Mettler-Toledo Columbus, OH, USA) | Fillers/film | According to ref [36] |
| Thermogravimetric analysis (TGA) | Mettler TGA/SDTA 851 (Mettler-Toledo Columbus, OH, USA) | Fillers/film | According to ref [36] |
| Dynamic Light Scattering (DLS) | Zetasizer ZSP (Malvern Panalytical Ltd. Malvern, U.K.) | Fillers | Refraction index (1.33–2.4) |
| Electrical conductivity measurement | HP 34401A Digital Multimeter (Keysight Technologies, Santa Rosa, Ca, USA) | Film (0.2 × 7.5 × 1 cm3) | 4-wire method According to ref [36] |
| Temperature measurement | -Thermocouples (Omega Engineering Ltd. Manchester U.K.) -Power supply (EA-PS 2042-20B) (EA Elektro-Automatik GmbH and Co.KG Helmholtzstr, Viersen) | Film (0.2 × 7.5 × 5 cm3) | According to ref [36] |
| Temperature distribution monitoring | Imager camera PCE-PI 450 (PCE Deutschland GmbH Meschede, Germany) | Film (0.2 × 7.5 × 5 cm3) | Frequency = 27 Hz IR resolution of 382 × 288 pixels |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadagno, L.; Foglia, F.; Pantani, R.; Romero-Sanchez, M.D.; Calderón, B.; Vertuccio, L. Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries. Nanomaterials 2020, 10, 1343. https://doi.org/10.3390/nano10071343
Guadagno L, Foglia F, Pantani R, Romero-Sanchez MD, Calderón B, Vertuccio L. Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries. Nanomaterials. 2020; 10(7):1343. https://doi.org/10.3390/nano10071343
Chicago/Turabian StyleGuadagno, Liberata, Fabiana Foglia, Roberto Pantani, Maria Dolores Romero-Sanchez, Blanca Calderón, and Luigi Vertuccio. 2020. "Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries" Nanomaterials 10, no. 7: 1343. https://doi.org/10.3390/nano10071343
APA StyleGuadagno, L., Foglia, F., Pantani, R., Romero-Sanchez, M. D., Calderón, B., & Vertuccio, L. (2020). Low-Voltage Icing Protection Film for Automotive and Aeronautical Industries. Nanomaterials, 10(7), 1343. https://doi.org/10.3390/nano10071343