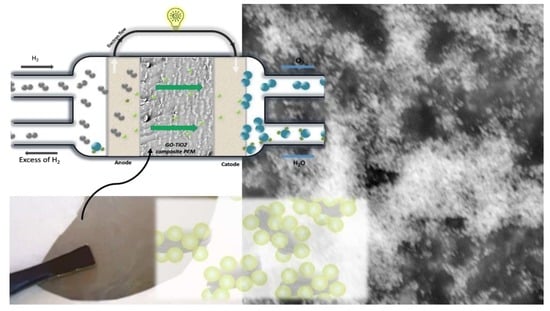
Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Graphene Oxide
2.3. Functionalization of GO with TiO2 (GO-TiO2)
2.4. Preparation of the Nanocomposite Membranes
2.5. Characterization of GO, GO-TiO2, and Composite Membranes
3. Results and Discussion
3.1. Graphene Oxide (GO) and GO-TiO2 Hybrid Nanomaterial
3.2. The sPSU-Based Nanocomposite Membranes
- (1)
- Water diffusion is higher in the nanocomposite membranes than in the filler-free sPSU;
- (2)
- The highest values are displayed by the sample at 3 wt.% of loading, which is also the membrane with the highest EIC and WU, confirming that agglomeration of the filler particles and occlusion of the hydrophilic cluster of sPSU occurs at higher loading;
- (3)
- The downfall of D at high temperatures is critical (and similar to the pristine membrane) for the composite sPSU_GO-TiO2 5%, while it is progressively less accentuated in the others, even becoming a plateau for the sPSU_GO-TiO2 3% membrane;
- (4)
- At 130 °C the D value in sPSU_GO-TiO2 3% is almost two order of magnitude higher than in the pristine sPSU, i.e., 1.1 × 10−5 and 1.3 × 10−7 cm2 s−1, respectively.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef] [PubMed]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC) e A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Inzelt, G.; Pineri, M.; Schultze, J.W.; Vorotyntsev, M.A. Electron and proton conducting polymers: Recent developments and prospects. Electrochim. Acta 2000, 45, 2403–2421. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.A.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications—A Review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Memb. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Nicotera, I. Transport Properties and Mechanical Features of Sulfonated Polyether Ether Ketone/Organosilica Layered Materials Nanocomposite Membranes for Fuel Cell Applications. Membranes 2020, 10, 87. [Google Scholar] [CrossRef]
- Kim, J.D.; Ohira, A.; Nakao, H. Chemically crosslinked sulfonated polyphenylsulfone (CSPPSU) membranes for PEM fuel cells. Membranes 2020, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Savinell, R.F.; Litt, M.H. Proton Conducting Polymers Used as Membranes. U.S. Patent 5,525,436, 6 November 1996. [Google Scholar]
- Nicotera, I.; Kosma, V.; Simari, C.; Angioni, S.; Mustarelli, P.; Quartarone, E. Ion dynamics and mechanical properties of sulfonated polybenzimidazole membranes for high-temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2015, 119, 9745–9753. [Google Scholar] [CrossRef]
- Donnadio, A.; Casciola, M.; Di Vona, M.L.; Tamilvanan, M. Conductivity and hydration of sulfonated polyethersulfone in the range 70–120 °C: Effect of temperature and relative humidity cycling. J. Power Sources 2012, 205, 145–150. [Google Scholar] [CrossRef]
- Simari, C.; Vecchio, C.L.; Enotiadis, A.; Davoli, M.; Baglio, V.; Nicotera, I. Toward optimization of a robust low-cost sulfonated-polyethersulfone containing layered double hydroxide for PEM fuel cells. J. Appl. Polym. Sci. 2019, 136, 47884. [Google Scholar] [CrossRef]
- De Bonis, C.; Simari, C.; Kosma, V.; Mecheri, B.; D’Epifanio, A.; Allodi, V.; Mariotto, G.; Brutti, S.; Suarez, S.; Pilar, K.; et al. Enhancement of proton mobility and mitigation of methanol crossover in sPEEK fuel cells by an organically modified titania nanofiller. J. Solid State Electrochem. 2016, 20, 1585–1598. [Google Scholar] [CrossRef]
- Lufrano, F.; Gatto, I.; Staiti, P.; Antonucci, V.; Passalacqua, E. Sulfonated polysulfone ionomer membranes for fuel cells. Solid State Ion. 2001, 145, 47–51. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Lo Vecchio, C.; Aricò, A.S.; Baglio, V.; Nicotera, I. Barrier properties of sulfonated polysulfone/layered double hydroxides nanocomposite membrane for direct methanol fuel cell operating at high methanol concentrations. Int. J. Hydrogen Energy 2020, 45, 20647–20658. [Google Scholar] [CrossRef]
- Yamada, O.; Yin, Y.; Tanaka, K.; Kita, H.; Okamoto, K.I. Polymer electrolyte fuel cells based on main-chain-type sulfonated polyimides. Electrochim. Acta 2005, 50, 2655–2659. [Google Scholar] [CrossRef]
- Laberty-Robert, C.; Vallé, K.; Pereira, F.; Sanchez, C. Design and properties of functional hybrid organic-inorganic membranes for fuel cells. Chem. Soc. Rev. 2011, 40, 961–1005. [Google Scholar] [CrossRef]
- Simari, C.; Lufrano, E.; Coppola, L.; Nicotera, I. Composite gel polymer electrolytes based on organo-modified nanoclays: Investigation on lithium-ion transport and mechanical properties. Membranes 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.S.; Schirmer, J.; Reissner, R.; Ruffmann, B.; Silva, H.; Mendes, A.; Madeira, L.M.; Nunes, S.P. Proton electrolyte membrane properties and direct methanol fuel cell performance—II. Fuel cell performance and membrane properties effects. J. Power Sources 2005, 140, 41–49. [Google Scholar] [CrossRef]
- Şengül, E.; Erdener, H.; Akay, R.G.; Yücel, H.; Baç, N.; Eroǧlu, I.I. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrogen Energy 2009, 34, 4645–4652. [Google Scholar]
- Chen, S.Y.; Han, C.C.; Tsai, C.H.; Huang, J.; Chen-Yang, Y.W. Effect of morphological properties of ionic liquid-templated mesoporous anatase TiO2 on performance of PEMFC with Nafion/TiO2 composite membrane at elevated temperature and low relative humidity. J. Power Sources 2007, 171, 363–372. [Google Scholar] [CrossRef]
- Hill, M.L.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Zirconium hydrogen phosphate/disulfonated poly(arylene ether sulfone) copolymer composite membranes for proton exchange membrane fuel cells. J. Memb. Sci. 2006, 283, 102–108. [Google Scholar] [CrossRef]
- Simari, C.; Enotiadis, A.; Lo Vecchio, C.; Baglio, V.; Coppola, L.; Nicotera, I. Advances in hybrid composite membranes engineering for high-performance direct methanol fuel cells by alignment of 2D nanostructures and a dual-layer approach. J. Memb. Sci. 2020, 599, 117858. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Simari, C.; Stallworth, P.; Peng, J.; Coppola, L.; Greenbaum, S.; Nicotera, I. Graphene oxide and sulfonated-derivative: Proton transport properties and electrochemical behavior of Nafion-based nanocomposites. Electrochim. Acta 2019, 297, 240–249. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, W.H.; Choi, B.S.; Kim, W.J. Preparation of Nafion/Pt-containing TiO2/graphene oxide composite membranes for self-humidifying proton exchange membrane fuel cell. J. Memb. Sci. 2016, 504, 20–28. [Google Scholar] [CrossRef]
- Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and Graphene Oxide for Fuel Cell Technology. Ind. Eng. Chem. Res. 2018, 57, 9333–9350. [Google Scholar] [CrossRef]
- Koduru, H.K.; Scarpelli, F.; Marinov, Y.G.; Hadjichristov, G.B.; Rafailov, P.M.; Miloushev, I.K.; Petrov, A.G.; Godbert, N.; Bruno, L.; Scaramuzza, N. Characterization of PEO/PVP/GO nanocomposite solid polymer electrolyte membranes: Microstructural, thermo-mechanical, and conductivity properties. Ionics (Kiel) 2018, 24, 3459–3473. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Enotiadis, A.; Angjeli, K.; Baldino, N.; Nicotera, I.; Gournis, D. Graphene-based nafion nanocomposite membranes: Enhanced proton transport and water retention by novel organo-functionalized graphene oxide nanosheets. Small 2012, 8, 3338–3349. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Bai, H.; Li, Y.; Zhang, H.; Chen, H.; Wu, W.; Wang, J.; Liu, J. Anhydrous proton exchange membranes comprising of chitosan and phosphorylated graphene oxide for elevated temperature fuel cells. J. Memb. Sci. 2015, 495, 48–60. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Zhang, H.; Zhang, T.; Zhang, B.; Cao, S.; Liu, J. Polydopamine-modified graphene oxide nanocomposite membrane for proton exchange membrane fuel cell under anhydrous conditions. J. Mater. Chem. A 2014, 2, 9548–9558. [Google Scholar] [CrossRef]
- Lanin, S.N.; Vlasenko, E.V.; Kovaleva, N.V.; Zung, F.T. The adsorption properties of titanium dioxide. Russ. J. Phys. Chem. A 2008, 82, 2152–2155. [Google Scholar] [CrossRef]
- Nicotera, I.; Khalfan, A.; Goenaga, G.; Zhang, T.; Bocarsly, A.; Greenbaum, S. NMR investigation of water and methanol mobility in nanocomposite fuel cell membranes. Ionics 2008, 14, 243–253. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Moszyński, D.; Kapica-Kozar, J.; Wanag, A.; Morawski, A.W. Assessment of the suitability of the one-step hydrothermal method for preparation of non-covalently/covalently-bonded TiO2/graphene-based hybrids. Nanomaterials 2018, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Cui, C.; Hub, H.; Wang, Y.; Xu, S.; Ying, B.; Li, P.; Lu, B.; Shen, H. One-step hydrothermal synthesis of anatase TiO2/reduced graphene oxide nanocomposites with enhanced photocatalytic activity. J. Alloys Compd. 2014, 582, 236–240. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P. V UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Lufrano, F.; Baglio, V.; Staiti, P.; Arico’, A.S.; Antonucci, V. Development and characterization of sulfonated polysulfone membranes for direct methanol fuel cells. Desalination 2006, 199, 283–285. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Agostini, M.; Enotiadis, A.; Brutti, S. A Novel Li + - Na fi on-Sulfonated Graphene Oxide Membrane as Single Lithium-Ion Conducting Polymer Electrolyte for Lithium Batteries. J. Phys. Chem. C 2019, 123, 27406–27416. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.S.; Sebastian, D.; Baglio, V. Sulfonated graphene oxide platelets in nafion nanocomposite membrane: Advantages for application in direct methanol fuel cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Nicotera, I.; Kosma, V.; Simari, C.; D’Urso, C.; Aricò, A.S.; Baglio, V. Methanol and proton transport in layered double hydroxide and smectite clay-based composites: Influence on the electrochemical behavior of direct methanol fuel cells at intermediate temperatures. J. Solid State Electrochem. 2015, 19, 2053–2061. [Google Scholar] [CrossRef]
- Tanner, J.E. Use of the stimulated echo in NMR diffusion studies. J. Chem Phys. 1970, 52, 2523–2526. [Google Scholar] [CrossRef]
- Simari, C.; Potsi, G.; Policicchio, A.; Perrotta, I.; Nicotera, I. Clay-Carbon Nanotubes Hybrid Materials for Nanocomposite Membranes: Advantages of Branched Structure for Proton Transport under Low Humidity Conditions in PEMFCs. J. Phys. Chem. C 2016, 120, 2574–2584. [Google Scholar] [CrossRef]
- Lian, Y.; Liu, Y.; Jiang, T.; Shu, J.; Lian, H.; Cao, M. Enhanced electromechanical performance of graphite oxide-nafion nanocomposite actuator. J. Phys. Chem. C 2010, 114, 9659–9663. [Google Scholar] [CrossRef]
- Burgess, R.; Buono, C.; Davies, P.R.; Davies, R.J.; Legge, T.; Lai, A.; Lewis, R.; Morgan, D.J.; Robinson, N.; Willock, D.J. The functionalisation of graphite surfaces with nitric acid: Identification of functional groups and their effects on gold deposition. J. Catal. 2015, 323, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.S.; Feng, W.J.; Lang, J.W.; Wang, R.T.; Tai, Z.X.; Yan, X. Bin Nitric acid modification of graphene nanosheets prepared by arc-discharge method and their enhanced electrochemical properties. Wuli Huaxue Xuebao Acta Phys. Chim. Sin. 2012, 28, 1726–1732. [Google Scholar]
- Carlucci, C.; Scremin, B.F.; Sibillano, T.; Giannini, C.; Filippo, E.; Perulli, P.; Capodilupo, A.L.; Corrente, G.A.; Ciccarella, G. Microwave-assisted synthesis of boron-modified TiO2 nanocrystals. Inorganics 2014, 2, 264–277. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Kuila, T.; Lee, J.H. Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J. Mater. Chem. A 2013, 1, 1349–1358. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Zeng, G.M.; Gong, J.L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B. Bin Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Kyzas, G.Z.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhao, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Enotiadis, A.; Nicotera, I.; Simari, C.; Charalambopoulou, G.; Giannelis, E.P.; Steriotis, T. Nafion® nanocomposite membranes with enhanced properties at high temperature and low humidity environments. Int. J. Hydrogen Energy 2016, 41, 22406–22414. [Google Scholar] [CrossRef] [Green Version]
- Enotiadis, A.; Boutsika, L.G.; Spyrou, K.; Simari, C.; Nicotera, I. A facile approach to fabricating organosilica layered material with sulfonic groups as an efficient filler for polymer electrolyte nanocomposites. New J. Chem. 2017, 41, 9489–9496. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Boutsika, L.G.; Coppola, L.; Spyrou, K.; Enotiadis, A. NMR investigation on nanocomposite membranes based on organosilica layered materials bearing different functional groups for PEMFCs. Int. J. Hydrogen Energy 2017, 42, 27940–27949. [Google Scholar] [CrossRef]
- Cozzi, D.; De Bonis, C.; D’Epifanio, A.; Mecheri, B.; Tavares, A.C.; Licoccia, S. Organically functionalized titanium oxide/Nafion composite proton exchange membranes for fuel cells applications. J. Power Sources 2014, 248, 1127–1132. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kulkarni, S.S. Ion Exchange Membranes: Preparation, Properties, and Applications; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789400717008. [Google Scholar]
- Cossari, P.; Simari, C.; Cannavale, A.; Gigli, G.; Nicotera, I. Advanced processing and characterization of Nafion electrolyte films for solid-state electrochromic devices fabricated at room temperature on single substrate. Solid State Ion. 2018, 317, 46–52. [Google Scholar] [CrossRef]
- Suarez, S.; Greenbaum, S. Nuclear magnetic resonance of polymer electrolyte membrane fuel cells. Chem. Rec. 2010, 10, 377–393. [Google Scholar] [CrossRef]
- Vasile, N.S.; Monteverde Videla, A.H.A.; Simari, C.; Nicotera, I.; Specchia, S. Influence of membrane-type and flow field design on methanol crossover on a single-cell DMFC: An experimental and multi-physics modeling study. Int. J. Hydrogen Energy 2017, 42, 27995–28010. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. Sulfonated graphene oxide-silica for highly selective Nafion-based proton exchange membranes. J. Mater. Chem. A 2014, 2, 16083–16092. [Google Scholar] [CrossRef]
- Rossi, C.O.; Caputo, P.; De Luca, G.; Maiuolo, L.; Eskandarsefat, S.; Sangiorgi, C. 1H-NMR spectroscopy: A possible approach to advanced bitumen characterization for industrial and paving applications. Appl. Sci. 2018, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Simari, C.; Baglio, V.; Lo Vecchio, C.; Aricò, A.S.; Agostino, R.G.; Coppola, L.; Oliviero Rossi, C.; Nicotera, I. Reduced methanol crossover and enhanced proton transport in nanocomposite membranes based on clay−CNTs hybrid materials for direct methanol fuel cells. Ionics 2017, 23, 2113–2123. [Google Scholar] [CrossRef]
- Li, G.H.; Lee, C.H.; Lee, Y.M.; Cho, C.G. Preparation of poly(vinyl phosphate-b-styrene) copolymers and its blend with PPO as proton exchange membrane for DMFC applications. Solid State Ion. 2006, 177, 1083–1090. [Google Scholar] [CrossRef]












| Membrane | IEC [meq g−1] | Water Uptake [%] |
|---|---|---|
| sPSU | 1.36 ± 0.01 | 27.2 ± 0.1 |
| sPSU_GO-TiO2 2% | 1.40 ± 0.02 | 31.6 ± 0.2 |
| sPSU_GO-TiO2 3% | 1.43 ± 0.02 | 33.4 ± 0.3 |
| sPSU_GO-TiO2 5% | 1.42 ± 0.01 | 31.8 ± 0.2 |
| Membrane | Proton Conductivity (mS cm−1) | |||
|---|---|---|---|---|
| 20% RH | 50% RH | 80% RH | 100% RH | |
| sPSU | 1.30 | 7.84 | 26.46 | 50.70 |
| sPSU_GO-TiO2 2% | 3.04 | 12.55 | 41.39 | 73.46 |
| sPSU_GO-TiO2 3% | 7.11 | 16.14 | 54.20 | 98.91 |
| sPSU_GO-TiO2 5% | 1.62 | 9.81 | 33.01 | 61.15 |
| Nafion 212 | 3.39 | 16.12 | 65.90 | 126.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simari, C.; Lufrano, E.; Godbert, N.; Gournis, D.; Coppola, L.; Nicotera, I. Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes. Nanomaterials 2020, 10, 1572. https://doi.org/10.3390/nano10081572
Simari C, Lufrano E, Godbert N, Gournis D, Coppola L, Nicotera I. Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes. Nanomaterials. 2020; 10(8):1572. https://doi.org/10.3390/nano10081572
Chicago/Turabian StyleSimari, Cataldo, Ernestino Lufrano, Nicolas Godbert, Dimitrios Gournis, Luigi Coppola, and Isabella Nicotera. 2020. "Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes" Nanomaterials 10, no. 8: 1572. https://doi.org/10.3390/nano10081572
APA StyleSimari, C., Lufrano, E., Godbert, N., Gournis, D., Coppola, L., & Nicotera, I. (2020). Titanium Dioxide Grafted on Graphene Oxide: Hybrid Nanofiller for Effective and Low-Cost Proton Exchange Membranes. Nanomaterials, 10(8), 1572. https://doi.org/10.3390/nano10081572