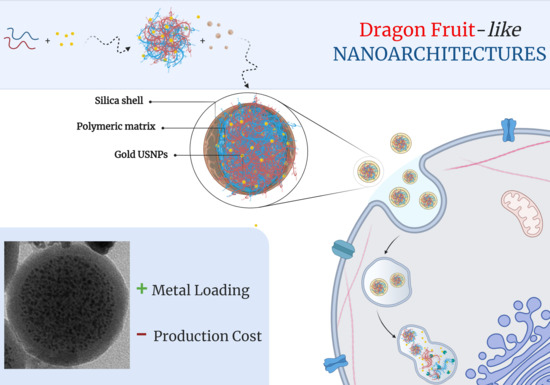
A Cost-Effective Approach for Non-Persistent Gold Nano-Architectures Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nano-Architectures
2.1.1. Synthesis of Dye-Modified Polyethyleneimine
2.1.2. Synthesis of Nano-Arrays
2.1.3. Synthesis of dNAs
2.2. Characterizations of Nano-Architectures
2.2.1. Dynamic Light Scattering (DLS) Measurements
2.2.2. UV–Vis Spectrophotometry
2.2.3. Fluorescence Spectrometry
2.2.4. Fourier Transform–Infrared (FT–IR) Spectroscopy
2.2.5. Electron Microscopy
2.2.6. Inductively Coupled Plasma–Mass Spectrometry (ICP–MS) Analysis
2.2.7. Silica Shell Dissolution Test
2.3. Cell Culture
2.3.1. Confocal Microscopy
2.3.2. Cell Viability Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlamidis, Y.; Voliani, V. Bringing again noble metal nanoparticles to the forefront of cancer therapy. Front. Bioeng. Biotechnol. 2018, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Mapanao, A.; Summa, M.; Vlamidis, Y.; Giannone, G.; Santi, M.; Guzzolino, E.; Pitto, L.; Poliseno, L.; Bertorelli, R.; et al. Biosafety and biokinetics of noble metals: The impact of their chemical nature. ACS Appl. Bio Mater. 2019, 2, 4464–4470. [Google Scholar] [CrossRef]
- Cassano, D.; Santi, M.; D’Autilia, F.; Mapanao, A.K.; Luin, S.; Voliani, V. Photothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures. Mater. Horiz. 2019, 6, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Her, S.; Borst, G.R.; Bristow, R.G.; Jaffray, D.A.; Allen, C. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother. Oncol. 2017, 124, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-nano approach: Enabling the translation of metal nanomaterials to clinics. Bioconjugate Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Giannone, G.; Summa, M.; Ermini, L.; Zamborlin, A.; Santi, M.; Cassano, D.; Bertorelli, R.; Voliani, V. Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2020, 1–6. [Google Scholar] [CrossRef]
- Cassano, D.; Summa, M.; Pocoví-Martínez, S.; Mapanao, A.K.; Catelani, T.; Bertorelli, R.; Voliani, V. Biodegradable ultrasmall-in-nano gold architectures: Mid-period in vivo distribution and excretion assessment. Part. Part. Syst. Charact. 2018, 36, 1800464. [Google Scholar] [CrossRef]
- Cassano, D.; Rota Martir, D.; Signore, G.; Piazza, V.; Voliani, V. Biodegradable hollow silica nanospheres containing gold nanoparticle arrays. Chem. Commun. 2015, 51, 9939–9941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapanao, A.K.; Santi, M.; Faraci, P.; Cappello, V.; Cassano, D.; Voliani, V. Endogenously triggerable ultrasmall-in-nano architectures: Targeting assessment on 3D pancreatic carcinoma spheroids. ACS Omega 2018, 3, 11796–11801. [Google Scholar] [CrossRef] [PubMed]
- Armanetti, P.; Pocoví-Martínez, S.; Flori, A.; Avigo, C.; Cassano, D.; Menichetti, L.; Voliani, V. Dual photoacoustic/ultrasound multi-parametric imaging from passion fruit-like nano-architectures. Nanomedicine 2018, 14, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- d’Amora, M.; Cassano, D.; Pocoví-Martínez, S.; Giordani, S.; Voliani, V. Biodistribution and biocompatibility of passion fruit-like nano-architectures in zebrafish. Nanotoxicology 2018, 12, 914–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A. Polyethylenimine in medicinal chemistry. Curr. Med. Chem. 2008, 15, 2826–2839. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Pocovı́-Martı́nez, S.; Cassano, D.; Voliani, V. Naked nanoparticles in Silica Nanocapsules: A versatile family of nanorattle catalysts. ACS Appl. Nano Mater. 2018, 1, 1836–1840. [Google Scholar] [CrossRef]
- van Bommel, K.J.C.; Jung, J.H.; Shinkai, S. Poly(L-lysine) aggregates as templates for the formation of hollow silica spheres. Adv. Mater. 2001, 13, 1472–1476. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Kind, L.; Shkilnyy, A.; Schlaad, H.; Meier, W.; Taubert, A. Poly(ethylene oxide)-poly(ethylene imine) block copolymers as templates and catalysts for the in situ formation of monodisperse silica nanospheres. Colloid Polym. Sci. 2010, 288, 1645–1650. [Google Scholar] [CrossRef]
- Masalov, V.M.; Sukhinina, N.S.; Kudrenko, E.A.; Emelchenko, G.A. Mechanism of formation and nanostructure of Stöber silica particles. Nanotechnology 2011, 22, 275718. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-sized CT contrast agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Al Zaki, A.; Joh, D.; Cheng, Z.; De Barros, A.L.B.; Kao, G.; Dorsey, J.; Tsourkas, A. Gold-Loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization. ACS Nano 2014, 8, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kunz-Schughart, L.A.; Dubrovska, A.; Peitzsch, C.; Ewe, A.; Aigner, A.; Schellenburg, S.; Muders, M.H.; Hampel, S.; Cirillo, G.; Iemma, F.; et al. Nanoparticles for radiooncology: Mission, vision, challenges. Biomaterials 2017, 120, 155–184. [Google Scholar] [CrossRef] [Green Version]
- Cassano, D.; Santi, M.; Cappello, V.; Luin, S.; Signore, G.; Voliani, V. Biodegradable passion fruit-like nano-architectures as carriers for cisplatin prodrug. Part. Part. Syst. Charact. 2016, 33, 818–824. [Google Scholar] [CrossRef]
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational design of a transferrin-binding peptide sequence tailored to targeted nanoparticle internalization. Bioconjugate Chem. 2017, 28, 471–480. [Google Scholar] [CrossRef]
- Santi, M.; Mapanao, A.K.; Cassano, D.; Vlamidis, Y.; Cappello, V.; Voliani, V. Endogenously-activated ultrasmall-in-nano therapeutics: Assessment on 3D head and neck squamous cell carcinomas. Cancers 2020, 12, 1063. [Google Scholar] [CrossRef]



| Nanoparticles Characterizations | NAs | dNAs |
|---|---|---|
| DLS Size [nm] | 203.1 ± 1.9 | 222.3 ± 6.3 |
| TEM Size [nm] | 107.6 ± 16.1 | 107.5 ± 16.6 |
| Shell thickness [nm] | 18.9 ± 2.2 | 12.0 ± 0.9 |
| Au loading [w/w%] | 5.9 ± 1.3 | 9.6 ± 0.8 |
| Z-potential [mV] | −20.6 ± 0.4 | −20.6 ± 0.6 |
| UV absorbance plasmon [nm] | 532 | 531 |
| Cell Line | Pearson’s Coefficient |
|---|---|
| SCC-25 | 0.82 ± 0.08 |
| UPCI:SCC154 | 0.66 ± 0.12 |
| MIA PaCa-2 | 0.86 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannone, G.; Santi, M.; Ermini, M.L.; Cassano, D.; Voliani, V. A Cost-Effective Approach for Non-Persistent Gold Nano-Architectures Production. Nanomaterials 2020, 10, 1600. https://doi.org/10.3390/nano10081600
Giannone G, Santi M, Ermini ML, Cassano D, Voliani V. A Cost-Effective Approach for Non-Persistent Gold Nano-Architectures Production. Nanomaterials. 2020; 10(8):1600. https://doi.org/10.3390/nano10081600
Chicago/Turabian StyleGiannone, Giulia, Melissa Santi, Maria Laura Ermini, Domenico Cassano, and Valerio Voliani. 2020. "A Cost-Effective Approach for Non-Persistent Gold Nano-Architectures Production" Nanomaterials 10, no. 8: 1600. https://doi.org/10.3390/nano10081600
APA StyleGiannone, G., Santi, M., Ermini, M. L., Cassano, D., & Voliani, V. (2020). A Cost-Effective Approach for Non-Persistent Gold Nano-Architectures Production. Nanomaterials, 10(8), 1600. https://doi.org/10.3390/nano10081600