Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting
Abstract
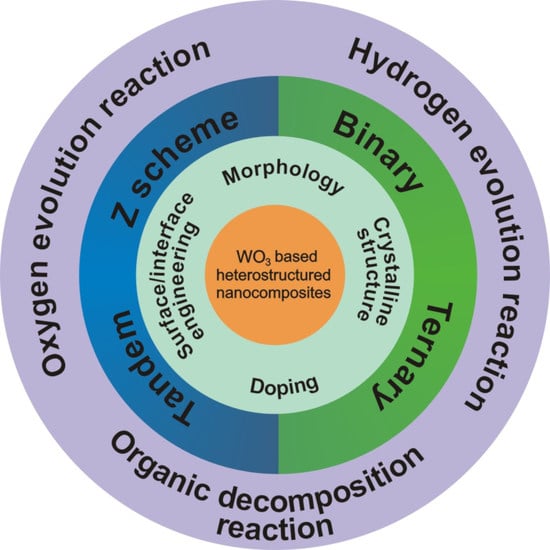
:1. Introduction
2. Basic Principles of the Water-Splitting Reaction
2.1. Thermodynamics of Water-Splitting
2.2. Device Requirements and Calculation of Their Efficiency
3. WO3 and Its Nanocomposites for Particle-Based Photocatalytic Systems
3.1. Half Reaction Systems
3.2. Z-Schemes
4. Heterostructured WO3 Nanocomposites for Photoelectrochemical Cell Systems
4.1. Crystalline Structure
4.2. Morphologic Effect
4.3. Binary Structures of Hierarchical Architectures Based on WO3 Semiconductors
4.3.1. Metal Oxide/Metal Oxide Binary Heterostructures
4.3.2. Metaloxide/Inorganic Compounds Heterostructures
4.3.3. Metal Oxide/Plasmon Particle Systems
4.4. Ternary Systems for Efficient Water Decomposition
4.5. WO3-Based Tandem PEC Cells
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sathre, R.; Scown, C.D.; Morrow, W.R.; Stevens, J.C.; Sharp, I.D.; Ager, J.W.; Walczak, K.; Houle, F.A.; Greenblatt, J.B. Life-cycle net energy assessment of large-scale hydrogen production via photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3264–3278. [Google Scholar] [CrossRef]
- Prevot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2012, 13, 14–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Hossain, M.F.; Takahashi, T. Photoelectrochemical water splitting on highly smooth and ordered TiO2 nanotube arrays for hydrogen generation. Int. J. Hydrog. Energy 2010, 35, 8528–8535. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Cesar, I.; Kay, A.; Gonzalez Martinez, J.A.; Grätzel, M. Translucent thin film Fe2O3 photoanodes for efficient water splitting by sunlight: Nanostructure-directing effect of Si-doping. J. Am. Chem. Soc. 2006, 128, 4582–4583. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Yin, M.; Fan, H.; Cheng, W.; Lu, L.; Song, Y.; Ma, J.; Zhu, X. Enhanced photoelectrocatalytic performance of α-Fe2O3 thin films by surface plasmon resonance of Au nanoparticles coupled with surface passivation by atom layer deposition of Al2O3. Nanoscale Res. Lett. 2015, 10, 374. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, R.; Zhu, J.; Shi, J.; Han, J.; Zong, X.; Li, C. Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: Essential relations between electrocatalyst and photocatalyst. J. Phys. Chem. C 2012, 116, 5082–5089. [Google Scholar] [CrossRef]
- Jo, W.J.; Jang, J.W.; Kong, K.-j.; Kang, H.J.; Kim, J.Y.; Jun, H.; Parmar, K.; Lee, J.S. Phosphate doping into monoclinic BiVO4 for enhanced photoelectrochemical water oxidation activity. Angew. Chem. 2012, 124, 3201–3205. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, H.; Zong, X.; Xu, Q.; Ma, Y.; Li, G.; Li, C. Crystal facet dependence of water oxidation on BiVO4 sheets under visible light irradiation. Chem. A Eur. J. 2011, 17, 1275–1282. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydrog. Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical study of nanostructured ZnO thin films for hydrogen generation from water splitting. Adv. Funct. Mater. 2009, 19, 1849–1856. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Solid solution of GaN and ZnO as a stable photocatalyst for overall water splitting under visible light. Chem. Mater. 2010, 22, 612–623. [Google Scholar] [CrossRef]
- Tacca, A.; Meda, L.; Marra, G.; Savoini, A.; Caramori, S.; Cristino, V.; Bignozzi, C.A.; Pedro, V.G.; Boix, P.P.; Gimenez, S. Photoanodes based on nanostructured WO3 for water splitting. ChemPhysChem 2012, 13, 3025–3034. [Google Scholar] [CrossRef]
- Hameed, A.; Gondal, M.; Yamani, Z. Effect of transition metal doping on photocatalytic activity of WO3 for water splitting under laser illumination: Role of 3d-orbitals. Catal. Commun. 2004, 5, 715–719. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A.; Schoonman, J. Study of photoactivity of tungsten trioxide (WO3) for water splitting. Thin Solid Films 2007, 515, 6371–6374. [Google Scholar] [CrossRef]
- Pala, R.A.; Leenheer, A.J.; Lichterman, M.; Atwater, H.A.; Lewis, N.S. Measurement of minority-carrier diffusion lengths using wedge-shaped semiconductor photoelectrodes. Energy Environ. Sci. 2014, 7, 3424–3430. [Google Scholar] [CrossRef] [Green Version]
- Coridan, R.H.; Arpin, K.A.; Brunschwig, B.S.; Braun, P.V.; Lewis, N.S. Photoelectrochemical behavior of hierarchically structured Si/WO3 core–shell tandem photoanodes. Nano Lett. 2014, 14, 2310–2317. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, H.; Cui, K.; Zhang, L.; Ge, S.; Jinghua, Y. Reversible electron storage in tandem photoelectrochemical cell for light driven unassisted overall water splitting. Appl. Catal. B Environ. 2020, 275, 119094. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Qiu, B. Enhanced surface charge separation induced by Ag nanoparticles on WO3 photoanode for photoelectrochemical water splitting. Chem. Lett. 2020, 49, 741–744. [Google Scholar] [CrossRef]
- Jun, J.; Ju, S.; Moon, S.; Son, S.; Huh, D.; Liu, Y.; Kim, K.; Lee, H. The optimization of surface morphology of Au nanoparticles on WO3 nanoflakes for plasmonic photoanode. Nanotechnology 2020, 31, 204003. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Ding, Y. Rationally designed/constructed CoOx/WO3 anode for efficient photoelectrochemical water oxidation. Acs Catal. 2017, 7, 1841–1845. [Google Scholar] [CrossRef]
- Wang, S.L.; Mak, Y.L.; Wang, S.; Chai, J.; Pan, F.; Foo, M.L.; Chen, W.; Wu, K.; Xu, G.Q. Visible–Near-Infrared-Light-Driven Oxygen Evolution Reaction with Noble-Metal-Free WO2–WO3 Hybrid Nanorods. Langmuir 2016, 32, 13046–13053. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 Double-Heterojunction photoanode with enhanced charge separation and visible-transparency for bias-free solar water-splitting with a perovskite solar cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wygant, B.R.; Mabayoje, O.; Lin, J.; Kawashima, K.; Kim, J.-H.; Li, W.; Li, J.; Mullins, C.B. Interface engineering and its effect on WO3-based photoanode and tandem cell. ACS Appl. Mater. Interfaces 2018, 10, 12639–12650. [Google Scholar] [CrossRef]
- Lee, W.J.; Shinde, P.S.; Go, G.H.; Ramasamy, E. Ag grid induced photocurrent enhancement in WO3 photoanodes and their scale-up performance toward photoelectrochemical H2 generation. Int. J. Hydrogen Energy 2011, 36, 5262–5270. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, G.; Liu, W.; Xi, Y.; Golosov, D.; Zavadski, S.; Melnikov, S. 3D core-shell WO3@ α-Fe2O3 photoanode modified by ultrathin FeOOH layer for enhanced photoelectrochemical performances. J. Alloys Compd. 2020, 154992. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, S.; Liu, B.K.; Wang, D.J.; Xie, T.F. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light. ACS Catal. 2014, 4, 3724–3729. [Google Scholar] [CrossRef]
- Lou, Z.; Zhu, M.; Yang, X.; Zhang, Y.; Whangbo, M.-H.; Li, B.; Huang, B. Continual injection of photoinduced electrons stabilizing surface plasmon resonance of non-elemental-metal plasmonic photocatalyst CdS/WO3− x for efficient hydrogen generation. Appl. Catal. B Environ. 2018, 226, 10–15. [Google Scholar] [CrossRef]
- Islam, S.Z.; Reed, A.; Wanninayake, N.; Kim, D.Y.; Rankin, S.E. Remarkable enhancement of photocatalytic water oxidation in N2/Ar plasma treated, mesoporous TiO2 films. J. Phys. Chem. C 2016, 120, 14069–14081. [Google Scholar] [CrossRef]
- Islam, S.Z.; Rankin, S.E. Hydrazine-based synergistic Ti (III)/N doping of surfactant-templated TiO2 thin films for enhanced visible light photocatalysis. Mater. Chem. Phys. 2016, 182, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.; Hu, X.; Ho, W.; Hu, P.; Huang, Y. Facile fabrication of porous Cr-doped SrTiO3 nanotubes by electrospinning and their enhanced visible-light-driven photocatalytic properties. J. Mater. Chem. A 2015, 3, 3935–3943. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Takanabe, K. Solar water splitting using semiconductor photocatalyst powders. In Solar Energy for Fuels; Springer: New York, NY, USA, 2015; pp. 73–103. [Google Scholar]
- Takanabe, K. Photocatalytic water splitting: Quantitative approaches toward photocatalyst by design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.-H.A.; Maeda, K.; Mallouk, T.E. Visible light water splitting using dye-sensitized oxide semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Eguchi, M.; Lee, S.-H.A.; Youngblood, W.J.; Hata, H.; Mallouk, T.E. Photocatalytic hydrogen evolution from hexaniobate nanoscrolls and calcium niobate nanosheets sensitized by ruthenium (II) bipyridyl complexes. J. Phys Chem. C 2009, 113, 7962–7969. [Google Scholar] [CrossRef]
- Islam, S.Z.; Reed, A.; Kim, D.Y.; Rankin, S.E. N2/Ar plasma induced doping of ordered mesoporous TiO2 thin films for visible light active photocatalysis. Microporous Mesoporous Mater. 2016, 220, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—the untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. A Chem. 2002, 148, 71–77. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Unal, U.; Tanaka, N.; Kudo, A.; Kato, H. Electrochemical approach to evaluate the mechanism of photocatalytic water splitting on oxide photocatalysts. J. Solid State Chem. 2004, 177, 4205–4212. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, G.; Tan, C.K.; Nguyen, T.D.; Tok, A.I.Y. Amorphous TiO2 coated hierarchical WO3 Nanosheet/CdS Nanorod arrays for improved photoelectrochemical performance. Appl. Surface Sc. 2019, 490, 411–419. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.-S. Effect of Electrolytes on the Selectivity and Stability of n-type WO3 Photoelectrodes for Use in Solar Water Oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Kato, H.; Sasaki, Y.; Iwase, A.; Kudo, A. Role of iron ion electron mediator on photocatalytic overall water splitting under visible light irradiation using Z-scheme systems. Bull. Chem. Soc. Jpn. 2007, 80, 2457–2464. [Google Scholar] [CrossRef]
- Miseki, Y.; Fujiyoshi, S.; Gunji, T.; Sayama, K. Photocatalytic water splitting under visible light utilizing I3−/I− and IO3−/I− redox mediators by Z-scheme system using surface treated PtO x/WO3 as O2 evolution photocatalyst. Catal. Sci. Technol. 2013, 3, 1750–1756. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Shang, T.; Chen, L.; Gu, L.; Peng, T. Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B Environ. 2017, 219, 693–704. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Luo, X.; Wen, J.; Ma, S.; Li, X.; Fang, Y.; Zhang, X. Enhanced visible light photocatalytic H2 production over Z-scheme g-C3N4 nansheets/WO3 nanorods nanocomposites loaded with Ni (OH)x cocatalysts. Chin. J. Catal. 2017, 38, 240–252. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Gao, R.; Zhang, Y.-C.; Pan, L.; Huang, C.; Liu, K.; Chang, X.-Y.; Zhang, X.; Zou, J.-J. Polarization-Enhanced direct Z-scheme ZnO-WO3-x nanorod arrays for efficient piezoelectric-photoelectrochemical Water splitting. Appl. Catal. B Environ. 2019, 259, 118079. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3−/I− shuttle redox mediator under visible light irradiation. Chem. Commun. 2001, 2416–2417. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Doping of WO3 for photocatalytic water splitting: Hints from density functional theory. J. Phys. Chem. C 2012, 116, 8901–8909. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, J.; Park, T.J.; Lee, J.S. Mg-doped WO3 as a novel photocatalyst for visible light-induced water splitting. Catal. Lett. 2002, 80, 53–57. [Google Scholar] [CrossRef]
- Higashimoto, S.; Ushiroda, Y.; Azuma, M. Electrochemically assisted photocatalysis of hybrid WO3/TiO2 films: Effect of the WO3 structures on charge separation behavior. Top. Catal. 2008, 47, 148–154. [Google Scholar] [CrossRef]
- Ke, D.; Liu, H.; Peng, T.; Liu, X.; Dai, K. Preparation and photocatalytic activity of WO3/TiO2 nanocomposite particles. Mater. Lett. 2008, 62, 447–450. [Google Scholar] [CrossRef]
- Cui, X.F.; Wang, Y.J.; Jiang, G.Y.; Zhao, Z.; Xu, C.M.; Wei, Y.C.; Duan, A.J.; Liu, J.; Gao, J.S. A photonic crystal-based CdS-Au-WO3 heterostructure for efficient visible-light photocatalytic hydrogen and oxygen evolution dagger. Rsc Adv. 2014, 4, 15689–15694. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Baek, M.; Kim, S.; Kim, W.; Yong, K. An antenna/spacer/reflector based Au/BiVO4/WO3/Au nanopatterned photoanode for plasmon-enhanced photoelectrochemical water splitting. Appl. Catal. B Environ. 2018, 237, 763–771. [Google Scholar] [CrossRef]
- Chatchai, P.; Murakami, Y.; Kishioka, S.-Y.; Nosaka, A.Y.; Nosaka, Y. Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation. Electrochim. Acta 2009, 54, 1147–1152. [Google Scholar] [CrossRef]
- Lee, M.G.; Kim, D.H.; Sohn, W.; Moon, C.W.; Park, H.; Lee, S.; Jang, H.W. Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation. Nano Energy 2016, 28, 250–260. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Asai, T.; Hisatomi, T.; Uemura, J.; Tosa, M.; Shimamura, K.; Kubota, J.; Domen, K. Nanostructured WO3/BiVO4 photoanodes for efficient photoelectrochemical water splitting. Small 2014, 10, 3692–3699. [Google Scholar] [CrossRef]
- Müller, A.; Kondofersky, I.; Folger, A.; Fattakhova-Rohlfing, D.; Bein, T.; Scheu, C. Dual absorber Fe2O3/WO3 host-guest architectures for improved charge generation and transfer in photoelectrochemical applications. Mater. Res. Express 2017, 4, 016409. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Chen, Y.; Qin, Z.; Wang, M.; Guo, L. Facile fabrication of sandwich structured WO3 nanoplate arrays for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 18089–18096. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Hwang, Y.J.; Chae, S.Y.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Fan, X.; Gao, B.; Wang, T.; Huang, X.; Gong, H.; Xue, H.; Guo, H.; Song, L.; Xia, W.; He, J. Layered double hydroxide modified WO3 nanorod arrays for enhanced photoelectrochemical water splitting. Appl. Catal. A General 2016, 528, 52–58. [Google Scholar] [CrossRef]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Joo, O.-S.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into charge separation in WO3/BiVO4 heterojunction for solar water splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef]
- Su, J.; Zhang, T.; Wang, L. Engineered WO3 nanorods for conformal growth of WO3/BiVO4 core–shell heterojunction towards efficient photoelectrochemical water oxidation. J. Mater. Sci. Mater. Electron. 2017, 28, 4481–4491. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Li, J.; Sun, D.; Chen, Q. Hydrothermal synthesis and photoelectrochemical properties of vertically aligned tungsten trioxide (hydrate) plate-like arrays fabricated directly on FTO substrates. J. Mater. Chem. 2012, 22, 17744–17752. [Google Scholar] [CrossRef]
- Amano, F.; Li, D.; Ohtani, B. Fabrication and photoelectrochemical property of tungsten (VI) oxide films with a flake-wall structure. Chem. Commun. 2010, 46, 2769–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Li, J.; Bai, J.; Li, X.; Xia, L.; Zhou, B. Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl. Catal. B Environ. 2017, 202, 388–396. [Google Scholar] [CrossRef]
- Nayak, A.K.; Sohn, Y.; Pradhan, D. Facile green synthesis of WO3 H2O nanoplates and WO3 nanowires with enhanced photoelectrochemical performance. Cryst. Growth Design 2017, 17, 4949–4957. [Google Scholar] [CrossRef]
- Park, M.; Seo, J.H.; Song, H.; Nam, K.M. Enhanced visible light activity of single-crystalline WO3 microplates for photoelectrochemical water oxidation. J. Phys. Chem. C 2016, 120, 9192–9199. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, G.; Wang, L.; Pan, J.; Lu, G.Q.M.; Cheng, H.-M. TiO2 films with oriented anatase {001} facets and their photoelectrochemical behavior as CdS nanoparticle sensitized photoanodes. J. Mater. Chem. 2011, 21, 869–873. [Google Scholar] [CrossRef]
- Zou, J.-P.; Wu, D.-D.; Luo, J.; Xing, Q.-J.; Luo, X.-B.; Dong, W.-H.; Luo, S.-L.; Du, H.-M.; Suib, S.L. A strategy for one-pot conversion of organic pollutants into useful hydrocarbons through coupling photodegradation of MB with photoreduction of CO2. Acs Catal. 2016, 6, 6861–6867. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Zhu, J.; Li, H.; Lu, Y. WO3 nanocrystals with tunable percentage of (0 0 1)-facet exposure. Appl. Catal. B Environ. 2012, 123, 398–404. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Gao, G.; Butburee, T.; Lyu, M.; Thaweesak, S.; Yun, J.-H.; Du, A.; Liu, G.; Wang, L. Synergistic crystal facet engineering and structural control of WO3 films exhibiting unprecedented photoelectrochemical performance. Nano Energy 2016, 24, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, P.; Wang, T.; Gong, J. Monoclinic WO3 nanomultilayers with preferentially exposed (002) facets for photoelectrochemical water splitting. Nano Energy 2015, 11, 189–195. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO 3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Kim, C.W.; Haider, Z.; Kang, Y.S. Facile fabrication of WO3 nanoplates thin films with dominant crystal facet of (002) for water splitting. Cryst. Growth Des. 2014, 14, 6057–6066. [Google Scholar] [CrossRef]
- Navarro, J.R.; Mayence, A.; Andrade, J.; Lerouge, F.; Chaput, F.; Oleynikov, P.; Bergstrom, L.; Parola, S.; Pawlicka, A. WO3 nanorods created by self-assembly of highly crystalline nanowires under hydrothermal conditions. Langmuir 2014, 30, 10487–10492. [Google Scholar] [CrossRef]
- Su, J.; Feng, X.; Sloppy, J.D.; Guo, L.; Grimes, C.A. Vertically aligned WO3 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis and photoelectrochemical properties. Nano Lett. 2011, 11, 203–208. [Google Scholar] [CrossRef]
- Rao, P.M.; Cho, I.S.; Zheng, X. Flame synthesis of WO3 nanotubes and nanowires for efficient photoelectrochemical water-splitting. Proc. Combust. Inst. 2013, 34, 2187–2195. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, N.-H.; Jang, J.-S.; Kim, I.-D. Catalyst-decorated hollow WO3 nanotubes using layer-by-layer self-assembly on polymeric nanofiber templates and their application in exhaled breath sensor. Sens. Actuators B Chem. 2016, 223, 301–310. [Google Scholar] [CrossRef]
- Chen, D.; Hou, X.; Wen, H.; Wang, Y.; Wang, H.; Li, X.; Zhang, R.; Lu, H.; Xu, H.; Guan, S. The enhanced alcohol-sensing response of ultrathin WO3 nanoplates. Nanotechnology 2009, 21, 035501. [Google Scholar] [CrossRef] [Green Version]
- Enferadi-Kerenkan, A.; Ello, A.S.; Do, T.-O. Synthesis, organo-functionalization, and catalytic properties of tungsten oxide nanoparticles as heterogeneous catalyst for oxidative cleavage of oleic acid as a model fatty acid into diacids. Ind. Eng. Chem. Res. 2017, 56, 10639–10647. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Kalkozova, Z.K.; Markhabayeva, A.A.; Dupre, R.; Moniruddin, M.; Nuraje, N. Core–Shell (W@ WO3) Nanostructure to Improve Electrochemical Performance. ACS Appl. Energy Mater. 2018, 2, 797–803. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Wang, S.; Wang, T.; Lian, J.; Duan, X.; Zheng, W. Topochemical preparation of WO3 nanoplates through precursor H2WO4 and their gas-sensing performances. J. Phys. Chem. C 2011, 115, 18157–18163. [Google Scholar] [CrossRef]
- Meng, D.; Wang, G.; San, X.; Song, Y.; Shen, Y.; Zhang, Y.; Wang, K.; Meng, F. Synthesis of WO3 flower-like hierarchical architectures and their sensing properties. J. Alloys Compd. 2015, 649, 731–738. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, D. Hydrothermal synthesis and electrochromism of WO3 nanocuboids. RSC Adv. 2014, 4, 20145–20153. [Google Scholar] [CrossRef]
- Ham, D.J.; Phuruangrat, A.; Thongtem, S.; Lee, J.S. Hydrothermal synthesis of monoclinic WO3 nanoplates and nanorods used as an electrocatalyst for hydrogen evolution reactions from water. Chem. Eng. J. 2010, 165, 365–369. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Sun, X.W.; Demir, H.V. Morphology-tailored synthesis of tungsten trioxide (hydrate) thin films and their photocatalytic properties. ACS Appl. Mater. Interfaces 2011, 3, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Nagy, D.; Nagy, D.; Szilágyi, I.M.; Fan, X. Effect of the morphology and phases of WO 3 nanocrystals on their photocatalytic efficiency. RSC Adv. 2016, 6, 33743–33754. [Google Scholar] [CrossRef] [Green Version]
- Sieb, N.R.; Wu, N.-c.; Majidi, E.; Kukreja, R.; Branda, N.R.; Gates, B.D. Hollow metal nanorods with tunable dimensions, porosity, and photonic properties. Acs Nano 2009, 3, 1365–1372. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 2012, 12, 1690–1696. [Google Scholar] [CrossRef]
- Kalanoor, B.S.; Seo, H.; Kalanur, S.S. Recent developments in photoelectrochemical water-splitting using WO3/BiVO4 heterojunction photoanode: A review. Mater. Sci. Energy Technol. 2018, 1, 49–62. [Google Scholar]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [Green Version]
- Hammad, A.; El-Bery, H.M.; El-Shazly, A.; Elkady, M. Effect of WO3 morphological structure on its photoelectrochemical properties. Int. J. Electrochem. Sci 2018, 13, 362–372. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Chen, Y.; Gaskov, A. Facile morphology control of WO3 nanostructure arrays with enhanced photoelectrochemical performance. Appl. Surface Sci. 2017, 403, 274–281. [Google Scholar] [CrossRef]
- Perera, D.; Lorek, R.; Khnayzer, R.S.; Moroz, P.; O’Connor, T.; Khon, D.; Diederich, G.; Kinder, E.; Lambright, S.; Castellano, F.N. Photocatalytic activity of core/shell semiconductor nanocrystals featuring spatial separation of charges. J. Phys. Chem. C 2012, 116, 22786–22793. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef]
- Jin, B.; Jung, E.; Ma, M.; Kim, S.; Zhang, K.; Kim, J.I.; Son, Y.; Park, J.H. Solution-processed yolk–shell-shaped WO3/BiVO4 heterojunction photoelectrodes for efficient solar water splitting. J. Mater. Chem. A 2018, 6, 2585–2592. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, G.; Wang, J.; Li, H.; Wu, J.; Du, Y. Refining waste hardmetals into tungsten oxide nanosheets via facile method. J. Nanoparticle Res. 2016, 18, 98. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Liu, P.; Shen, Y.; Cheng, C.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Versatile nanoporous bimetallic phosphides towards electrochemical water splitting. Energy Environ. Sci. 2016, 9, 2257–2261. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, D.; Kim, Y.; Lee, J. Highly donor-doped (110) layered perovskite materials as novel photocatalysts for overall water splitting. Chem. Commun. 1999, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Wang, L.; Kado, Y.; Killian, M.S.; Schmuki, P. Anodic nanotubular/porous hematite photoanode for solar water splitting: Substantial effect of iron substrate purity. ChemSusChem 2014, 7, 934–940. [Google Scholar] [CrossRef]
- Troitskaia, I.; Gavrilova, T.; Atuchin, V. Structure and micromorphology of titanium dioxide nanoporous microspheres formed in water solution. Phys. Procedia 2012, 23, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Liu, Y.; Chen, S.; Shi, J.; Wang, J.; Fan, A.; Zan, W.; Li, S.; Goddard, W.A.; Zhang, X.-M. Defect-enriched iron fluoride-oxide nanoporous thin films bifunctional catalyst for water splitting. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Han, H.S.; Kim, J.S.; Park, I.J.; Lee, M.H.; Hong, K.S.; Cho, I.S. A tree-like nanoporous WO3 photoanode with enhanced charge transport efficiency for photoelectrochemical water oxidation. J. Mater. Chem. A 2015, 3, 12920–12926. [Google Scholar] [CrossRef]
- Fujimoto, I.; Wang, N.; Saito, R.; Miseki, Y.; Gunji, T.; Sayama, K. WO3/BiVO4 composite photoelectrode prepared by improved auto-combustion method for highly efficient water splitting. Int. J. Hydrogen Energy 2014, 39, 2454–2461. [Google Scholar] [CrossRef]
- Choi, K.-S. Shape effect and shape control of polycrystalline semiconductor electrodes for use in photoelectrochemical cells. J. Phys. Chem. Lett. 2010, 1, 2244–2250. [Google Scholar] [CrossRef]
- Song, K.; Gao, F.; Yang, W.; Wang, E.; Wang, Z.; Hou, H. WO3 mesoporous nanobelts towards efficient photoelectrocatalysts for water splitting. ChemElectroChem 2018, 5, 322–327. [Google Scholar] [CrossRef]
- Markhabayeva, A.A.; Moniruddin, M.; Dupre, R.; Abdullin, K.A.; Nuraje, N. Designing of WO3@ Co3O4 Heterostructure to Enhance Photoelectrochemical Performances. J. Phys. Chem. A 2020, 124, 486–491. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Vayssieres, L.; Shen, S.; Guo, L.; Wheeler, D.A.; Zhang, J.Z.; Antoun, B.R.; Mao, S.S. A perspective on solar-driven water splitting with all-oxide hetero-nanostructures. Energy Environ. Sci. 2011, 4, 3889–3899. [Google Scholar] [CrossRef]
- Moniruddin, M.; Afroz, K.; Shabdan, Y.; Bizri, B.; Nuraje, N. Hierarchically 3D assembled strontium titanate nanomaterials for water splitting application. Appl. Surface Sci. 2017, 419, 886–892. [Google Scholar] [CrossRef]
- Shabdan, Y.; Ronasi, A.; Coulibaly, P.; Moniruddin, M.; Nuraje, N. Engineered core-shell nanofibers for electron transport study in dye-sensitized solar cells. AIP Adv. 2017, 7, 065008. [Google Scholar] [CrossRef] [Green Version]
- Abdullin, K.A.; Azatkaliev, A.; Gabdullin, M.; Kalkozova, Z.K.; Mukashev, B.; Serikkanov, A. Preparation of Nanosized Tungsten and Tungsten Oxide Powders. Phys. Solid State 2018, 60, 2634–2639. [Google Scholar] [CrossRef]
- Lisitsyna, L.; Denisov, G.; Dauletbekova, A.; Karipbayev, Z.T.; Markhabaeva, A.; Vaganov, V.; Lisitsyn, V.M.; Akilbekov, A. Luminescence of LiF crystals doped with uranium. Proc. J. Phys. Conf. Ser. 2017, 830, 012156. [Google Scholar] [CrossRef]
- Moniruddin, M.; Oppong, E.; Stewart, D.; McCleese, C.; Roy, A.; Warzywoda, J.; Nuraje, N. Designing CdS-Based Ternary Heterostructures Consisting of Co-Metal and CoOx Cocatalysts for Photocatalytic H2 Evolution under Visible Light. Inorg. Chem. 2019, 58, 12325–12333. [Google Scholar] [CrossRef] [PubMed]
- De Tacconi, N.R.; Chenthamarakshan, C.; Rajeshwar, K.; Pauporté, T.; Lincot, D. Pulsed electrodeposition of WO3–TiO2 composite films. Electrochem. Commun. 2003, 5, 220–224. [Google Scholar] [CrossRef]
- Smith, W.; Wolcott, A.; Fitzmorris, R.C.; Zhang, J.Z.; Zhao, Y. Quasi-core-shell TiO2/WO3 and WO3/TiO2 nanorod arrays fabricated by glancing angle deposition for solar water splitting. J. Mater. Chem. 2011, 21, 10792–10800. [Google Scholar] [CrossRef]
- Bae, S.W.; Ji, S.M.; Hong, S.J.; Jang, J.W.; Lee, J.S. Photocatalytic overall water splitting with dual-bed system under visible light irradiation. Int. J. Hydrogen Energy 2009, 34, 3243–3249. [Google Scholar] [CrossRef]
- Ohno, T.; Tanigawa, F.; Fujihara, K.; Izumi, S.; Matsumura, M. Photocatalytic oxidation of water on TiO2-coated WO3 particles by visible light using Iron (III) ions as electron acceptor. J. Photochem. Photobiol. A Chem. 1998, 118, 41–44. [Google Scholar] [CrossRef]
- Yang, M.; He, H.; Zhang, H.; Zhong, X.; Dong, F.; Ke, G.; Chen, Y.; Du, J.; Zhou, Y. Enhanced photoelectrochemical water oxidation on WO3 nanoflake films by coupling with amorphous TiO2. Electrochim. Acta 2018, 283, 871–881. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Davarzadeh, M. Single-step electrochemical anodization for synthesis of hierarchical WO3–TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J. Electroanal. Chem. 2015, 739, 149–155. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Preparation of hybrid WO3–TiO2 nanotube photoelectrodes using anodization and wet impregnation: Improved water-splitting hydrogen generation performance. Int. J. Hydrogen Energy 2013, 38, 2156–2166. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Song, K.Y.; Lee, W.I.; Choi, G.J.; Do, Y.R. Photocatalytic behavior of WO3-loaded TiO2 in an oxidation reaction. J. Catal. 2000, 191, 192–199. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. Comparative studies of operational parameters of degradation of azo dyes in visible light by highly efficient WOx/TiO2 photocatalyst. J. Hazard. Mater. 2010, 177, 781–791. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Bai, J.; Li, J.; Zeng, Q.; Li, X.; Zhou, B. A highly efficient BiVO4/WO3/W heterojunction photoanode for visible-light responsive dual photoelectrode photocatalytic fuel cell. Appl. Catal. B Environ. 2016, 183, 224–230. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Grigioni, I.; Stamplecoskie, K.G.; Selli, E.; Kamat, P.V. Dynamics of photogenerated charge carriers in WO3/BiVO4 heterojunction photoanodes. J. Phys. Chem. C 2015, 119, 20792–20800. [Google Scholar] [CrossRef]
- Pattengale, B.; Ludwig, J.; Huang, J. Atomic insight into the W-doping effect on carrier dynamics and photoelectrochemical properties of BiVO4 photoanodes. J. Phys. Chem. C 2016, 120, 1421–1427. [Google Scholar] [CrossRef]
- Mali, M.G.; Yoon, H.; Kim, M.-w.; Swihart, M.T.; Al-Deyab, S.S.; Yoon, S.S. Electrosprayed heterojunction WO3/BiVO4 films with nanotextured pillar structure for enhanced photoelectrochemical water splitting. Appl. Phys. Lett. 2015, 106, 151603. [Google Scholar] [CrossRef]
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Graätzel, M. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 2008, 113, 772–782. [Google Scholar] [CrossRef]
- Sadtler, B.; Demchenko, D.O.; Zheng, H.; Hughes, S.M.; Merkle, M.G.; Dahmen, U.; Wang, L.-W.; Alivisatos, A.P. Selective facet reactivity during cation exchange in cadmium sulfide nanorods. J. Am. Chem. Soc. 2009, 131, 5285–5293. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, L.; Liu, R.; Cao, Z.; Sun, X.; Liu, X.; Luo, J. WO3@ α-Fe2O3 Heterojunction arrays with improved photoelectrochemical behavior for neutral ph water splitting. ChemCatChem 2016, 8, 2765–2770. [Google Scholar] [CrossRef]
- Luo, W.; Yu, T.; Wang, Y.; Li, Z.; Ye, J.; Zou, Z. Enhanced photocurrent–voltage characteristics of WO3/Fe2O3 nano-electrodes. J. Phys. D Appl. Phys. 2007, 40, 1091. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Li, H.; Wei, X.; Wang, R.; Zhou, A. Photoelectrochemical splitting of natural seawater with α-Fe2O3/WO3 nanorod arrays. Int. J. Hydrogen Energy 2016, 41, 4096–4105. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Reddy, G.B. Core-Shell WO3-WS2 Nanostructured Thin Films via Plasma Assisted Sublimation and Sulfurization. ACS Appl. Nano Mater. 2019, 2, 1691–1703. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, Z. Novel WO3/Sb2S3 heterojunction photocatalyst based on WO3 of different morphologies for enhanced efficiency in photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 9684–9691. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Chen, L.; Cao, F.; Guo, J.; Li, L. Three-dimensional WO3 nanoplate/Bi2S3 nanorod heterojunction as a highly efficient photoanode for improved photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2017, 9, 40235–40243. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Hu, D.; Diao, P.; Xu, D.; Wu, Q. Gold/WO3 nanocomposite photoanodes for plasmonic solar water splitting. Nano Res. 2016, 9, 1735–1751. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic solar water splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Thimsen, E.; Le Formal, F.; Gratzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe2O3 electrodes for water splitting. Nano Lett. 2010, 11, 35–43. [Google Scholar] [CrossRef]
- Singh, T.; Müller, R.; Singh, J.; Mathur, S. Tailoring surface states in WO3 photoanodes for efficient photoelectrochemical water splitting. Appl. Surface Sci. 2015, 347, 448–453. [Google Scholar] [CrossRef]
- Li, Y.Z.; Liu, Z.; Guo, Z.; Ruan, M.; Li, X.; Liu, Y. Efficient WO3 photoanode modified by Pt layer and plasmonic Ag for enhanced charge separation and transfer to promote photoelectrochemical performances. ACS Sustain. Chem. Eng. 2019, 7, 12582–12590. [Google Scholar]
- Xu, S.; Fu, D.; Song, K.; Wang, L.; Yang, Z.; Yang, W.; Hou, H. One-dimensional WO3/BiVO4 heterojunction photoanodes for efficient photoelectrochemical water splitting. Chem. Eng. J. 2018, 349, 368–375. [Google Scholar] [CrossRef]
- Wu, P.D.; Liu, Z.F.; Ruan, M.N.; Guo, Z.G.; Zhao, L. Cobalt-phosphate modified Fe-Zn0.2Cd0.8S/CuSbS2 heterojunction photoanode with multiple synergistic effect for enhancing photoelectrochemical water splitting. Appl. Surface Sci. 2019, 476, 716–723. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, J.; Kim, W.; Choi, W. Enhanced Photocatalytic and Photoelectrochemical Activity in the Ternary Hybrid of CdS/TiO2/WO3 through the Cascadal Electron Transfer. J. Phys. Chem. C 2011, 115, 9797–9805. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Bai, J.; Li, J.H.; Li, L.S.; Xia, L.G.; Zhou, B.X.; Sun, Y.G. Highly-stable and efficient photocatalytic fuel cell based on an epitaxial TiO2/WO3/W nanothorn photoanode and enhanced radical reactions for simultaneous electricity production and wastewater treatment. Appl. Energy 2018, 220, 127–137. [Google Scholar] [CrossRef]
- Liu, C.H.; Qiu, Y.Y.; Zhang, J.; Liang, Q.; Mitsuzaki, N.; Chen, Z.D. Construction of CdS quantum dots modified g-C3N4/ZnO heterostructured photoanode for efficient photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2019, 371, 109–117. [Google Scholar] [CrossRef]
- Liew, S.L.; Zhang, Z.; Goh, T.W.G.; Subramanian, G.S.; Seng, H.L.D.; Hor, T.S.A.; Luo, H.K.; Chi, D.Z. Yb-doped WO3 photocatalysts for water oxidation with visible light. Int. J. Hydrogen Energy 2014, 39, 4291–4298. [Google Scholar] [CrossRef]
- Subramanyam, P.; Vinodkumar, T.; Nepak, D.; Deepa, M.; Subrahmanyam, C. Mo-doped BiVO4@reduced graphene oxide composite as an efficient photoanode for photoelectrochemical water splitting. Catal. Today 2019, 325, 73–80. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.J.; Kim, J.K.; Park, J.H. Photoelectrochemical cells with tungsten trioxide/Mo-doped BiVO4 bilayers. Phys. Chem. Chem. Phys. 2012, 14, 11119–11124. [Google Scholar] [CrossRef]
- Tian, L.; Yang, X.F.; Cui, X.K.; Liu, Q.Q.; Tang, H. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance. Appl. Surface Sci. 2019, 463, 9–17. [Google Scholar] [CrossRef]
- Kim, J.Y.; Youn, D.H.; Kang, K.; Lee, J.S. Highly Conformal Deposition of an Ultrathin FeOOH Layer on a Hematite Nanostructure for Efficient Solar Water Splitting. Angew. Chem. Int. Ed. 2016, 55, 10854–10858. [Google Scholar] [CrossRef] [PubMed]
- Lhermitte, C.R.; Verwer, J.G.; Bartlett, B.M. Improving the stability and selectivity for the oxygen-evolution reaction on semiconducting WO3 photoelectrodes with a solid-state FeOOH catalyst. J. Mater. Chem. A 2016, 4, 2960–2968. [Google Scholar] [CrossRef]
- Bai, S.L.; Yang, X.J.; Liu, C.Y.; Xiang, X.; Luo, R.X.; He, J.; Chen, A.F. An Integrating Photoanode of WO3/Fe2O3 Heterojunction Decorated with NiFe-LDH to Improve PEC Water Splitting Efficiency. Acs Sustain. Chem. Eng. 2018, 6, 12906–12913. [Google Scholar] [CrossRef]
- Davi, M.; Ogutu, G.; Schrader, F.; Rokicinska, A.; Kustrowski, P.; Slabon, A. Enhancing Photoelectrochemical Water Oxidation Efficiency of WO3/alpha-Fe2O3 Heterojunction Photoanodes by Surface Functionalization with CoPd Nanocrystals. Eur. J. Inorg. Chem. 2017, 2017, 4267–4274. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Yang, X.; Bai, S.; Luo, R.; Li, D.; Chen, A. Photoanode of coupling semiconductor heterojunction and catalyst for solar PEC water splitting. Electrochim. Acta 2020, 331, 135282. [Google Scholar] [CrossRef]
- Tahir, M.; Siraj, M.; Tahir, B.; Umer, M.; Alias, H.; Othman, N. Au-NPs embedded Z–scheme WO3/TiO2 nanocomposite for plasmon-assisted photocatalytic glycerol-water reforming towards enhanced H2 evolution. Appl. Surface Sci. 2020, 503, 144344. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Yan, Y.; Li, J.Q. Preparation of flower-like BiOBr-WO3-Bi2WO6 ternary hybrid with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 651, 184–192. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.P.; Liu, J.K.; Feng, P.Y. Branched WO3 nanosheet array with layered C3N4 heterojunctions and CoOx Nanoparticles as a flexible photoanode for efficient photoelectrochemical water oxidation. Adv. Mater. 2014, 26, 5043–5049. [Google Scholar] [CrossRef]
- Yan, H.J.; Tian, C.G.; Wang, L.; Wu, A.P.; Meng, M.C.; Zhao, L.; Fu, H.G. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 6325–6329. [Google Scholar] [CrossRef]
- Liu, C.J.; Yang, Y.H.; Li, W.Z.; Li, J.; Li, Y.M.; Shi, Q.L.; Chen, Q.Y. Highly Efficient Photoelectrochemical Hydrogen Generation Using ZnxBi2S3+x Sensitized Platelike WO3 Photoelectrodes. Acs Appl. Mater. Interfaces 2015, 7, 10763–10770. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Ren, L.L.; Guo, Y.G.; Liang, H.P.; Cao, A.M.; Wan, L.J.; Bai, C.L. Mass production and high photocatalytic activity of ZnS nanoporous nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, X.; Wang, D.F.; Ye, J.H. Conformal BiVO4-Layer/WO3-Nanoplate-Array Heterojunction Photoanode Modified with Cobalt Phosphate Cocatalyst for Significantly Enhanced Photoelectrochemical Performances. Acs Appl. Mater. Interfaces 2019, 11, 5623–5631. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zhang, X.T.; Yuan, B.; Wang, Y.X.; Sun, P.P.; Wang, D.; Wei, Y.A.; Liu, Y.C. Multi-heterojunction photocatalysts based on WO3 nanorods: Structural design and optimization for enhanced photocatalytic activity under visible light. Chem. Eng. J. 2014, 237, 29–37. [Google Scholar] [CrossRef]
- Ran, L.; Yin, L.W. Ternary Hierarchical Cu7S4/TiO2/CoCr-LDH Heterostructured Nanorod Arrays with Multiphase Reaction Interfaces for More Efficient Photoelectrochemical Water Splitting. Adv. Mater. Interfaces 2019, 6, 14. [Google Scholar] [CrossRef]
- Song, S.Y.; Zhang, Y.; Xing, Y.; Wang, C.; Feng, J.; Shi, W.D.; Zheng, G.L.; Zhang, H.J. Rectangular AgIn(WO4)(2) nanotubes: A promising photoelectric material. Adv. Funct. Mater. 2008, 18, 2328–2334. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Hou, H.L.; Song, K.; Fang, Z.; Wang, L.; Gao, F.M.; Yang, Z.B.; Tang, B.; Yang, W.Y. Ternary WO3/Porous-BiVO4/FeOOH Hierarchical Architectures: Towards Highly Efficient Photoelectrochemical Performance. Chemelectrochem 2018, 5, 3660–3667. [Google Scholar] [CrossRef]
- Shi, X.J.; Choi, Y.; Zhang, K.; Kwon, J.; Kim, D.Y.; Lee, J.K.; Oh, S.H.; Kim, J.K.; Park, J.H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures. Nat. Commun. 2014, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Beermann, N.; Vayssieres, L.; Lindquist, S.E.; Hagfeldt, A. Photoelectrochemical studies of oriented nanorod thin films of hematite. J. Electrochem. Soc. 2000, 147, 2456–2461. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dai, C.H.; Liang, Q.M.; Yang, P.; Yang, H.H.; Yan, J.H. Constructing ZnO/ZnCr2O4@TiO2-NTA Nanocomposite for Photovoltaic Conversion and Photocatalytic Hydrogen Evolution. J. Electron. Mater. 2019, 48, 1724–1729. [Google Scholar] [CrossRef]
- Cai, J.J.; Li, S.; Qin, G.W. Interface engineering of Co3O4 loaded CaFe2O4/Fe2O3 heterojunction for photoelectrochemical water oxidation. Appl. Surface Sci. 2019, 466, 92–98. [Google Scholar] [CrossRef]
- Yu, C.L.; Chen, F.Y.; Zeng, D.B.; Xie, Y.; Zhou, W.Q.; Liu, Z.; Wei, L.F.; Yang, K.; Li, D.H. A facile phase transformation strategy for fabrication of novel Z-scheme ternary heterojunctions with efficient photocatalytic properties. Nanoscale 2019, 11, 7720–7733. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhao, Z.Y.; Li, Z.S.; Ye, J.H.; Zou, Z.G. Preparation and photocatalytic property of LiCr(WO4)(2). J. Alloys Compd. 2009, 485, 346–350. [Google Scholar] [CrossRef]
- Coridan, R.H.; Shaner, M.; Wiggenhorn, C.; Brunschwig, B.S.; Lewis, N.S. Electrical and Photoelectrochemical Properties of WO3/Si Tandem Photoelectrodes. J. Phys. Chem. C 2013, 117, 6949–6957. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Turner, J.A. Characterization of Hematite Thin Films for Photoelectrochemical Water Splitting in a Dual Photoelectrode Device. J. Electrochem. Soc. 2010, 157, F173–F178. [Google Scholar] [CrossRef]
- Wang, H.L.; Deutsch, T.; Turner, J.A. Direct water splitting under visible light with nanostructured hematite and WO3 photoanodes and a GaInP2 photocathode. J. Electrochem. Soc. 2008, 155, F91–F96. [Google Scholar] [CrossRef] [Green Version]
- Fountaine, K.T.; Atwater, H.A. Mesoscale modeling of photoelectrochemical devices: Light absorption and carrier collection in monolithic, tandem, Si vertical bar WO3 microwires. Opt. Express 2014, 22, A1453–A1461. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Shen, S.H.; Wang, M.; Ren, F.; Liu, Y.; Zheng, X.D.; Liu, Y.C.; Xiao, X.H.; Wu, W.; Jiang, C.Z. Efficient enhancement of solar-water-splitting by modified “Z-scheme” structural WO3-W-Si photoelectrodes. Appl. Phys. Lett. 2014, 105, 143902. [Google Scholar] [CrossRef]
- Sherman, B.D.; Sheridan, M.V.; Wee, K.R.; Marquard, S.L.; Wang, D.G.; Alibabaei, L.; Ashford, D.L.; Meyer, T.J. A Dye-Sensitized Photoelectrochemical Tandem Cell for Light Driven Hydrogen Production from Water. J. Am. Chem. Soc. 2016, 138, 16745–16753. [Google Scholar] [CrossRef]
- Shi, X.; Jeong, H.; Oh, S.J.; Ma, M.; Zhang, K.; Kwon, J.; Choi, I.T.; Choi, I.Y.; Kim, H.K.; Kim, J.K.; et al. Unassisted photoelectrochemical water splitting exceeding 7% solar-to-hydrogen conversion efficiency using photon recycling. Nat. Commun. 2016, 7, 11943. [Google Scholar] [CrossRef]
- Akple, M.S.; Chimmikuttanda, S.P. A ternary Z-scheme WO3-Pt-CdS composite for improved visible-light photocatalytic H-2 production activity. J. Nanoparticle Res. 2018, 20, 16. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Lee, S.A.; Suh, J.M.; Hong, S.-P.; Jang, H.W. Triple Planar Heterojunction of SnO2/WO3/BiVO4 with Enhanced Photoelectrochemical Performance under Front Illumination. Appl. Sci. Basel 2018, 8, 1765. [Google Scholar] [CrossRef] [Green Version]
- Yourey, J.E.; Kurtz, J.B.; Bartlett, B.M. Water Oxidation on a CuWO4-WO3 Composite Electrode in the Presence of Fe(CN)(6) (3-): Toward Solar Z-Scheme Water Splitting at Zero Bias. J. Phys. Chem. C 2012, 116, 3200–3205. [Google Scholar] [CrossRef]
- Prasad, U.; Prakash, J.; Gupta, S.K.; Zuniga, J.; Mao, Y.B.; Azeredo, B.; Kannan, A.N.M. Enhanced Photoelectrochemical Water Splitting with Er- and W-Codoped Bismuth Vanadate with WO3 Heterojunction-Based Two-Dimensional Photoelectrode. Acs Appl. Mater. Interfaces 2019, 11, 19029–19039. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhang, H.; Yang, Y.; Cheng, C. 3D Brochosomes-Like TiO2/WO3/BiVO4 Arrays as Photoanode for Photoelectrochemical Hydrogen Production. Small 2019, 15, 924. [Google Scholar] [CrossRef]
- Ma, W.; Wu, X.; Huang, K.; Wang, M.; Fu, R.; Chen, H.; Feng, S. A Co(OH)(x) nanolayer integrated planar WO3/Fe2O3 photoanode for efficient photoelectrochemical water splitting. Sustain. Energy Fuels 2019, 3, 2135–2141. [Google Scholar] [CrossRef]
- Salimi, R.; Alvani, A.A.S.; Mei, B.T.; Naseri, N.; Du, S.F.; Mul, G. Ag-Functionalized CuWO4/WO3 nanocomposites for solar water splitting. New J. Chem. 2019, 43, 2196–2203. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Xia, M.Y.; Li, C.; Yue, C.S.; Diao, P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts 2018, 8, 663. [Google Scholar] [CrossRef] [Green Version]
- Leonard, K.C.; Nam, K.M.; Lee, H.C.; Kang, S.H.; Park, H.S.; Bard, A.J. ZnWO4/WO3 Composite for Improving Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 15901–15910. [Google Scholar] [CrossRef]
- Scarongella, M.; Gadiyar, C.; Strach, M.; Rimoldi, L.; Loiudice, A.; Buonsanti, R. Assembly of -Cu2V2O7/WO3 heterostructured nanocomposites and the impact of their composition on structure and photoelectrochemical properties. J. Mater. Chem. C 2018, 6, 12062–12069. [Google Scholar] [CrossRef]
- Li, K.Z.; Zhang, C.; Liu, A.J.; Chu, D.M.; Zhang, C.Y.; Yang, P.; Du, Y.K.; Huang, J. Mesoporous tungsten oxide modified by nanolayered manganese-calcium oxide as robust photoanode for solar water splitting. J. Colloid Interface Sci. 2018, 516, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Liu, Z.F.; Zhang, J.; Guo, Z.G.; Xin, Y.; Zhao, L. 1D/0D WO3/CdS heterojunction photoanodes modified with dual co-catalysts for efficient photoelectrochemical water splitting. J. Alloy. Compd. 2019, 790, 493–501. [Google Scholar] [CrossRef]
- Cui, Y.; Pan, L.; Chen, Y.; Afzal, N.; Ullah, S.; Liu, D.Y.; Wang, L.; Zhang, X.W.; Zou, J.J. Defected ZnWO4-decorated WO3 nanorod arrays for efficient photoelectrochemical water splitting. Rsc. Adv. 2019, 9, 5492–5500. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.Z.; Song, K.; Wang, L.; Gao, F.M.; Tang, B.; Hou, H.L.; Yang, W.Y. WO3/BiVO4 Type-II Heterojunction Arrays Decorated with Oxygen-Deficient ZnO Passivation Layer: A Highly Efficient and Stable Photoanode. Acs Appl. Mater. Interfaces 2019, 11, 889–897. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y.K.; Chen, C.M.; Wang, T.H.; Zhang, M. Octopus tentacles-like WO3/C@CoO as high property and long life-time electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 281, 1–8. [Google Scholar] [CrossRef]
- Yuan, K.P.; Cao, Q.; Li, X.Y.; Chen, H.Y.; Deng, Y.H.; Wang, Y.Y.; Luo, W.; Lu, H.L.; Zhang, D.W. Synthesis of WO3@ZnWO4@ZnO-ZnO hierarchical nanocactus arrays for efficient photoelectrochemical water splitting. Nano Energy 2017, 41, 543–551. [Google Scholar] [CrossRef]
- Lin, H.S.; Lin, L.Y. Improving Visible-light Responses and Electric Conductivities by Incorporating Sb2S3 and Reduced Graphene Oxide in a WO3 Nanoplate Array for Photoelectrochemical Water Oxidation. Electrochim. Acta 2017, 252, 235–244. [Google Scholar] [CrossRef]
- Jamali, S.; Moshaii, A. Improving photo-stability and charge transport properties of Cu2O/CuO for photo-electrochemical water splitting using alternate layers of WO3 or CuWO4 produced by the same route. Appl. Surface Sci. 2017, 419, 269–276. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhou, W.W.; Yang, Y.P.; Cheng, C.W. 3D WO3/BiVO4/Cobalt Phosphate Composites Inverse Opal Photoanode for Efficient Photoelectrochemical Water Splitting. Small 2017, 13, 8. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.H.; Park, J.; Seo, H. Insights into the electronic bands of WO3/BiVO4/TiO2, revealing high solar water splitting efficiency. J. Mater. Chem. A 2017, 5, 1455–1461. [Google Scholar] [CrossRef]
- Gao, H.Q.; Zhang, P.; Hu, J.H.; Pan, J.M.; Fan, J.J.; Shao, G.S. One-dimenSional Z-scheme TiO2/WO3/Pt heterostructures for enhanced hydrogen generation. Appl. Surface Sci. 2017, 391, 211–217. [Google Scholar] [CrossRef]
- Peng, X.N.; He, C.; Liu, Q.Y.; Wang, X.N.; Wang, H.B.; Zhang, Y.J.; Ma, Q.C.; Zhang, K.; Han, Y.B.; Wang, H. Strategic Surface Modification of TiO2 nanorods by WO3 and TiCl4 for the Enhancement in Oxygen Evolution Reaction. Electrochim. Acta 2016, 222, 1112–1119. [Google Scholar] [CrossRef]















| Photoanodes | Methods | Morphology | Electrolyte | Potential | P (mW/cm2) | J (mA/cm2)/STH (%)/Gas Evolution (mL/cm2) | Ref |
|---|---|---|---|---|---|---|---|
| WO3/TiO2 | Solvothermal | Nanoflake | 0.1-M Na2SO4 | 0.8 vs. SCE (1.45 vs. RHE) | 100 | 1.4 | [129] |
| WO3/TiO2 | Anodization | Nanotubes | 1-M NaOH | 0.7 vs. Ag/AgCl (1.7 RHE) | 100 | 1.6 | [130] |
| WO3/TiO2 | Anodization | Nanotubes | 1-M KOH | 0.6 V vs. SCE (1.62 RHE) | 100 | 2 /3.1%/16.2 | [131] |
| WO3−x/ZnO | Solvothermal method | Nanorods | 1-M Na2SO4 | 1.23 vs. RHE | 100 | 3.38 | [56] |
| WO3/BiVO4 | Glancing-angle deposition/electrodeposition | Vertically oriented nanorods | 0.5-M Na2SO4 | 1.23 vs. RHE | 100 | 3.1 | [66] |
| WO3/BiVO4 | Electrostaticspraying method | Nanotextured pillar | 0.5-M Na2SO4 | 0.7 V vs. Ag/AgCl (1.44 vs. RHE) | 100 | 2.1 | [139] |
| WO3/BiVO4 | Layer-by-layer | Film | 0.5-M Na2SO4 | 1.23 vs. Ag/AgCl (1.817 vs. RHE) | 100 | 2.78 | [134] |
| WO3/BiVO4 | Spin coating | Film | 0.5-M Na2SO4 | 1.23 V vs. Ag/AgCl 1.817 vs. RHE | 100 | 1.2 | [64] |
| WO3/BiVO4 | Pulsed electrodeposition | Nanorods | 0.1-M Na2SO3 | 1.23 vs. RHE | 100 | 4.55 | [65] |
| WO3/BiVO4 | Anodic oxidation | Nanoporous film | 0.1-M KH2PO4 | 0.6 V vs. Ag/AgCl (1.21 vs. RHE) | 100 | 02.01 | [135] |
| WO3/BiVO4 | Electrospinning | Nanofibers | 0.5-M Na2SO4 | 1.23 vs. RHE | 100 | 2.8 | [154] |
| WO3/Fe2O3 | Solvothermal | Nanosheets | 0.5-M Na2SO4 | 1.23 vs. RHE | 100 | 1.66 | [142] |
| WO3/Fe2O3 | Hydrothermal | Nanorods | 0.1-M Na2SO4 | 1.23 vs. RHE | 100 | 1 | [144] |
| WO3/Fe2O3 | Sol–gel | Film | 0.1-M NaOH | 1.23 vs. RHE | 100 | 0.7 | [67] |
| WO3/Sb2S3 | Hydrothermal | Nanoplate/nanorods | 1-M H2SO4 | 0.8 vs. RHE | 100 | 1.79 | [146] |
| WO3/Bi2S3 | CBD | Nanorods/nanoplates | 0.1-M Na2SO3 | 0.9 vs. RHE | 100 | 5.95 | [147] |
| WO3/Au | Hydrothermal | Nanoplate | 0.1-M Na2SO4 | 1.23 vs. RHE | 100 | 1.5 | [149] |
| Photocatalytic Material | Methods | Morphology | Electrolyte | Potential | Irradiation | Photocurrent Density | Ref |
|---|---|---|---|---|---|---|---|
| WO3–Pt–CdS | Combination of wet-chemical, photodeposition and hydrothermal techniques | Hollow microspheres composed of small crystallites | 0.5 M Na2SO4 | 0.5 V vs. Ag/AgCl (1.82 V vs. RHE) | Vis light | 0.16 μA/cm2 | [192] |
| SnO2/WO3/BiVO4 | Combination of electron beam deposition and metal organic decomposition technique | Planar film | 0.5-M Na2SO3 | 1.23 vs. RHE | 100 mW/cm2 | 2.01 mA/cm2 | [193] |
| WO3/C3N 4//CoOx | Combination of a hydrothermal method with wet impregnation | film | 1.23 V vs. Ag/AgCl (1.8 V vs. RHE) | 100 mW/cm2 | 5.76 mA/cm2 | [170] | |
| CuWO4−WO3 | electrodeposition | film | 0.1-M KH2PO4 | 0.618 V vs. Ag/AgCl (1.23 vs. RHE) | 100 mW/cm2 | 0.3 mA/cm2 | [194] |
| WO3/(Er, W):BiVO4 | spray coating | monoclinic clinobisvanite structure | 0.1-M K2HPO4 | 1.23 V vs. RHE | 100 mW/cm2 | 4.1 ± 0.19 mA cm−2 | [195] |
| WO3/(Er, W):BiVO4 | spray coating | monoclinic clinobisvanite structure | 0.1-M K2HPO4 | 2.3 V vs. RHE | 100 mW/cm2 | 7.2 ± 0.39 mA cm−2 | [195] |
| TiO2/WO3/BiVO4 | hydrothermal | brochosomes-like | 0.5-M Na2SO4 | 0.35 V vs. RHE | 100 mW/cm2 | 3.13 mA cm−2 | [196] |
| WO3/ Fe2O3/Co(OH) | electrospray deposition | worm-like nanobars | 0.1-M NaOH | 1.23 vs. RHE | 0.62 mA cm−2 | [197] | |
| Ag-functionalized CuWO4/WO3 | electrophoretic deposition | thin film | potassium phosphate buffer solution | 0.62 V vs. Ag/AgCl (1.23 V vs. RHE) | 0.205 mA cm−2 | [198] | |
| CuWO4/BiVO4 with Co-Pi | drop-casting and thermal annealing method | nanoflakes | 1.0 M of Na2SO4 with 0.1 M of sodium phosphate buffer (pH = 7) | 1.23 V vs. RHE | 100 mW/cm2 | 2.25 mA cm−2 | [199] |
| BiVO4/WO3/SnO2 connected with perovskite solar cell tandem device | Spin-coating | triple-layer planar film | pH 7 phosphate buffer electrolyte | 1.23 V vs. RHE | 100 mW/cm2 | 3.1 mA/cm2 | [26] |
| ZnWO4/WO3 | Piezo-dispensing | Spot Arrays | 0.1-M Na2SO4 at pH 7 | 0.7 V vs. Ag/AgCl (1.31 V vs. RHE) | 0.75 mA/cm2 | [200] | |
| b-Cu2V2O7/WO3 | Seeded-growth approach | 0.1-M sodium borate buffer (pH 8.2) containing 0.1-M Na2SO3 | 1.23 V vs. RHE | 100 mW/cm2 | 0.45 mA cm−2 | [201] | |
| CaMn2O4/WO3 | Spin-coating | Thin film | 0.5-M Na2SO4 solution (pH 6) | 1.09 V vs. RHE | 1.5 × 10−3 mA cm−2 | [202] | |
| Pt/WO3/Ag | Hydrothermal method, chemical bath, photoassisted electrodeposition | Nanorods | 100 mW/cm2 | 1.13 mA/cm2 | [153] | ||
| WO3/CdS/NiOOH | hydrothermal method, successive ionic layer adsorption and reaction, photo-assisted electrodeposition | Nanorods | d 0.2-M Na2SO4-0.1-M Na2SO3 | 1.23 V vs. RHE | 1.5–2 mA/cm2 | [203] | |
| ZnWO4/WO3 | hydrothermal | Nanorods | 0.5 M Na2SO4 | 1.23 V vs. RHE | 100 mW/cm2 | 1.87 mA cm−2 | [204] |
| WO3/BiVO4/ZnO | drop-casting method, atomic layer deposition | Nanosheets | 0.5-M Na2SO4 | 1.23 V vs. RHE | 100 mW/cm2 | 2.5–3.00 mA cm−2 | [205] |
| Au-surface/BiVO4/WO3/Au-bottom | hydrothermal, sol–gel spin-coated, | Nanospheres | 0.5 M Na2SO4 | 1.23 V vs. RHE | 1.31 mA/cm2 | [63] | |
| WO3/C@CoO | hydrothermal process and immersion method | Octopus tentacles-like | 1.0-M KOH | 55 mV (vs. RHE) | 10 mA cm−2 | [206] | |
| WO3@ZnWO4@ZnO | layer deposition technique and hydrothermal process | nanosheets | mixed aqueous solution of 0.35-M Na2S and 0.25-M NaSO3 (pH = 13.4) | 1.23 V vs. RHE | 100 mW/cm2 | ~1.57 mA/cm2 | [207] |
| WO3/rGO/Sb2S3 | chemical bath deposition | nanoplates | 0.5-M Na2SO4 (pH~7) | 1.23 V vs. RHE | 1.20 mA/cm2 | [208] | |
| Cu2O/CuO/WO3 | Electrodeposition, spin-coating | Thin film | 0 V vs. RHE | −1.9 mA/cm2 | [209] | ||
| WO3/BiVO4/Co-Pi | Electrodeposition | composite inverse opals | 0.5-M Na2SO4 | 1.4 V versus Ag/AgCl (0.67 V vs. RHE) | 100 mW cm−2 | 4.5 mA cm−2 | [210] |
| WO3/BiVO4/TiO2 | Spin-coating, wet chemistry | platelike | 0.1-M Na2SO4 | 1.23 V vs. RHE | 100 mW/cm2 | ~3.9 mA/cm2 | [211] |
| TiO2/WO3/Pt | Electrospinning technique | fibers | 0.2-M Na2SO4 | 15–20×10−3 mA/cm2 | [212] | ||
| TiO2-TiCl4-WO3 | Hydrothermal method + Electrodeposition | nanorods | KOH | 1.23 V vs NHE | 100 mW/cm2 | 3.86 mW/cm2 | [213] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabdan, Y.; Markhabayeva, A.; Bakranov, N.; Nuraje, N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials 2020, 10, 1871. https://doi.org/10.3390/nano10091871
Shabdan Y, Markhabayeva A, Bakranov N, Nuraje N. Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials. 2020; 10(9):1871. https://doi.org/10.3390/nano10091871
Chicago/Turabian StyleShabdan, Yerkin, Aiymkul Markhabayeva, Nurlan Bakranov, and Nurxat Nuraje. 2020. "Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting" Nanomaterials 10, no. 9: 1871. https://doi.org/10.3390/nano10091871
APA StyleShabdan, Y., Markhabayeva, A., Bakranov, N., & Nuraje, N. (2020). Photoactive Tungsten-Oxide Nanomaterials for Water-Splitting. Nanomaterials, 10(9), 1871. https://doi.org/10.3390/nano10091871