Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds
Abstract
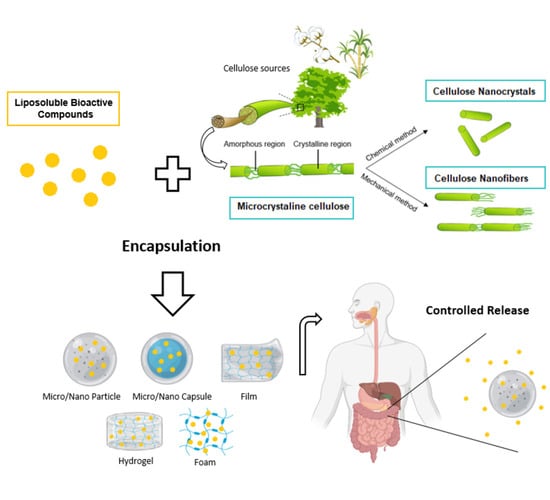
:1. Introduction
2. Cellulose-Based Materials
2.1. Cellulose Basics: Sources, Isolation Methods, and Structural Features
2.2. Cellulose Supramolecular Structures
2.2.1. Microcrystalline Cellulose—MCC
2.2.2. Cellulose Nanocrystals—CNC
2.2.3. Cellulose Nanofibers—CNF
3. Challenges of Liposoluble Compounds Delivery
4. Cellulose Systems for Encapsulation and Controlled Release of Liposoluble Compounds
4.1. Microcrystalline Cellulose
4.2. Cellulose Nanocrystals
4.3. Cellulose Nanofibers
5. Safety and Potential Toxicity of Cellulose Micro and Nanostructures
6. Digestibility, Bioaccessibility, and Bioavailability
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Balmayor, E.R.; Azevedo, H.S.; Reis, R.L. Controlled delivery systems: From pharmaceuticals to cells and genes. Pharm. Res. 2011, 28, 1241–1258. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2018, 59, 3129–3151. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, T.; Millstone, E. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A review. J. Microencapsul. 2015, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.L.; Tan, C.R.; Lim, P.D.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. Surface-modified nanocrystalline cellulose from oil palm empty fruit bunch for effective binding of curcumin. Int. J. Biol. Macromol. 2019, 138, 1064–1071. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Kolakovic, R. Nanofibrillar Cellulose in Drug Delivery; Faculty of Pharmacy of the University of Helsinki: Helsinki, Finland, 2013. [Google Scholar]
- Svagan, A.J.; Benjamins, J.-W.; Al-Ansari, Z.; Bar Shalom, D.; Müllertz, A.; Wågberg, L.; Löbmann, K. Solid cellulose nanofiber based foams—Towards facile design of sustained drug delivery systems. J. Control. Release 2016, 244, 74–82. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011, 9, 2140. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Jafari, S.M.; McClements, D.J. Nanotechnology approaches for increasing nutrient bioavailability. In Advances in Food and Nutrition Research; Steve, T., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2017; pp. 1–30. [Google Scholar]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef]
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery. In Nanocellulose and Nanohydrogel Matrices; Jawaid, M., Mohammad, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 1–19. [Google Scholar]
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-Layer Assembled Thin Films and Microcapsules of Nanocrystalline Cellulose for Hydrophobic Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Martins, J.T.; Duarte, C.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Letchford, K.; Jackson, J.K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Pickering stabilized nanocellulose-alginate: A diosgenin-mediated delivery of quinalizarin as a potent cyto-inhibitor in human lung/breast cancer cell lines. Mater. Sci. Eng. C 2020, 109, 110621. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 1–14. [Google Scholar] [CrossRef]
- Sun, L.; Yang, S.; Qian, X.; An, X. Cyclodextrin and cellulose combination product developed by click chemistry: Fascinating design for inclusion of ciprofloxacin. Cellulose 2020, 27, 5955–5970. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A. Enhancing the functional properties of acetylated hemicellulose films for active food packaging using acetylated nanocellulose reinforcement and polycaprolactone coating. Food Packag. Shelf Life 2020, 24, 100481. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Antimicrobial and wound treatment aspects of micro- and Nanoformulations of Carbox-ymethyl, Dialdehyde, and TEMPO-oxidized derivatives of cellulose: Recent advances. Macromol. Biosci. 2020, 4, 1900362. [Google Scholar]
- Taheri, A.; Mohammadi, M. The use of cellulose nanocrystals for potential application in topical delivery of hydroquinone. Chem. Biol. Drug Des. 2014, 86, 102–106. [Google Scholar] [CrossRef]
- Sarkar, G.; Orasugh, J.T.; Saha, N.R.; Roy, I.; Bhattacharyya, A.; Chattopadhyay, A.K.; Rana, D.; Chattopadhyay, D. Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J. Chem. 2017, 41, 15312–15319. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crop. Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- El Abasy, A.; Shoaib, A.; Waqas, M.; Shi, Z.; Jiang, M. Cellulose Nanocrystals loaded with thiamethoxam: Fabrication, characterization, and evaluation of insecticidal activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Nanomaterials 2020, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Abo-Elseoud, W.S.; Hassan, M.L.; Sabaa, M.W.; Basha, M.; Hassan, E.A.; Fadel, S.M. Use of cellulose nanocrystals in chitosan nanoparticles carrier system for the controlled release of ketoprofen. OSP J. Nanomed. Nanotechnol. 2020, 1, 1–11. [Google Scholar]
- Supramaniam, J.; Adnan, R.; Kaus, N.H.M.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of Cellulose-based Materials in Sustained Drug Delivery Systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef]
- Xie, J.; Li, J. Smart drug delivery system based on nanocelluloses. J. Bioresour. Bioprod. 2017, 2, 1–3. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Gonçalves, G.A.B. Synthesis and Characterization of TiO2 Cellulose Nanocomposites. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2007. [Google Scholar]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Motloung, M.P.; Ojijo, V.; Bandyopadhyay, J.; Ray, S.S. Cellulose Nanostructure-Based Biodegradable Nanocomposite Foams: A Brief Overview on the Recent Advancements and Perspectives. Polymers 2019, 11, 1270. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, Q.; Chen, W.; Liu, Y.; Yu, H. Self-Assembly of Nanocellulose and indomethacin into hierarchically ordered structures with high encapsulation efficiency for sustained release applications. ChemPlusChem 2014, 79, 725–731. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-Mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Morán, J.I.; Álvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2010, 18, 451–459. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- de Oliveira, F.B.; Bras, J.; Pimenta, M.T.B.; Curvelo, A.A.D.S.; Belgacem, M.N. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Ind. Crop. Prod. 2016, 93, 48–57. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Kale, R.D.; Bansal, P.S.; Gorade, V. Extraction of microcrystalline cellulose from cotton sliver and its comparison with commercial microcrystalline cellulose. J. Polym. Environ. 2017, 26, 355–364. [Google Scholar] [CrossRef]
- Lam, N.T.; Saewong, W.; Sukyai, P. Effect of varying hydrolysis time on extraction of spherical bacterial cellulose nanocrystals as a reinforcing agent for poly(vinyl alcohol) composites. J. Polym. Res. 2017, 24, 71. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, W.; Chen, L.; Lan, W.; Zhu, J.Y.; Runge, T. A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 2015, 23, 493–503. [Google Scholar] [CrossRef]
- Otsuka, I.; Njinang, C.N.; Borsali, R. Simple fabrication of cellulose nanofibers via electrospinning of dissolving pulp and tunicate. Cellulose 2017, 24, 3281–3288. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline nanocellulose—preparation, modification, and properties. In Cellulose—Fundamental Aspects and Current Trends; InTech Open: Rijeka, Croatia, 2015; Volume 9, pp. 159–191. [Google Scholar]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Radotić, K.; Mićić, M. Methods for Extraction and Purification of Lignin and Cellulose from Plant Tissues; Humana Press: New York, NY, USA, 2016; pp. 365–376. [Google Scholar]
- Maryana, R.; Ma’rifatun, D.; Wheni, A.I.; Satriyo, K.W.; Rizal, W.A. Alkaline pretreatment on sugarcane bagasse for bioethanol production. In Energy Procedia; Elsevier: Amsterdam, The Netherlands, 2014; pp. 250–254. [Google Scholar]
- de Carvalho, D.M.; Sevastyanova, O.; de Queiroz, J.H.; Colodette, J.L. Cold alkaline extraction as a pretreatment for bioethanol production from eucalyptus, sugarcane bagasse and sugarcane straw. Energy Convers. Manag. 2016, 124, 315–324. [Google Scholar] [CrossRef]
- Schell, D.J.; Farmer, J.; Newman, M.; McMillan, J.D. Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor: Investigation of yields, kinetics, and enzymatic digestibilities of solids. In Applied Biochemistry and Biotechnology—Part A Enzyme Engineering and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 69–86. [Google Scholar]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Pin, T.C.; Nakasu, P.; Mattedi, S.; Rabelo, S.; Costa, A.C. Screening of protic ionic liquids for sugarcane bagasse pretreatment. Fuel 2018, 235, 1506–1514. [Google Scholar] [CrossRef]
- Rieland, J.M.; Love, B.J. Ionic liquids: A milestone on the pathway to greener recycling of cellulose from biomass. Resour. Conserv. Recycl. 2020, 155, 104678. [Google Scholar] [CrossRef]
- Fan, J.; De Bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct Microwave-Assisted Hydrothermal Depolymerization of Cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Janardhnan, S.; Sain, M.M. Cellulose Microfibril Isolation—An enzymatic approach. BioResources 2006, 1, 176–188. [Google Scholar] [CrossRef]
- Zhu, Z.; Simister, R.; Bird, S.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Microwave assisted acid and alkali pretreatment of Miscanthus biomass for biorefineries. AIMS Environ. Sci. 2015, 2, 449–468. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: Optimization through response surface methodology. Bioresour. Technol. 2012, 112, 293–299. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. Microcrystalline cellulose production from sugarcane bagasse: Sustainable process development and life cycle assessment. J. Clean. Prod. 2019, 249, 119342. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, X.; You, T.T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Qu, Y.; Yin, W.; Zhang, R.; Zhao, S.; Liu, L.; Yu, J. Isolation and characterization of cellulosic fibers from ramie using organosolv degumming process. Cellulose 2019, 27, 1225–1237. [Google Scholar] [CrossRef]
- Gan, S.; Zakaria, S.; Chen, R.S.; Chia, C.H.; Padzil, F.N.M.; Moosavi, S. Autohydrolysis processing as an alternative to enhance cellulose solubility and preparation of its regenerated bio-based materials. Mater. Chem. Phys. 2017, 192, 181–189. [Google Scholar] [CrossRef]
- Bassani, A.; Fiorentini, C.; Vellingiri, V.; Flavio, M.; Spigno, G. Autohydrolysis of wheat straw for antioxidants and cellulosic Fiber Recovery. In Proceedings of the 5th European Congress of Applied Biotechnology, Florence, Italy, 15–19 September 2019; pp. 1690–1691. [Google Scholar]
- Nanta, P.; Kasemwong, K.; Skolpap, W.; Shimoyama, Y. Influence of supercritical carbon dioxide treatment on the physicochemical properties of cellulose extracted from cassava pulp waste. J. Supercrit. Fluids 2019, 154, 104605. [Google Scholar] [CrossRef]
- Hebeish, A.; Guthrie, T.J. The Chemistry and Technology of Cellulosic Copolymers; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing fibrillated cellulose as a sustainable technological material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Tan, T.H.; Lee, H.V.; Dabdawb, W.A.Y.; Abd Hamid, S.B.B.O. A Review of Nanocellulose in the Drug-Delivery System; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Singh, A.A.; Khan, M.J.; Ansari, M.A.; Farooqi, H.; Svedberg, A.; Karim, Z. Nanocellulose and nanohydrogel matrices as sustainable biomass materials: Structure, properties, present status, and future prospects in construction and other engineering. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Mohammad, F., Al-lohedan, H.A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–195. [Google Scholar]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- TAPPI—Technical Association of the Pulp and Paper Industry. WI 3021—Standard Terms and Their Definition for Cellulose Nanomaterials; Tappi: Peachtree Corners, GA, USA, 2012. [Google Scholar]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline Cellulose as Pharmaceutical Excipient. In Pharmaceutical Formulation Design-Recent Practices; Ahmad, U., Akhtar, J., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Carlin, B. Direct compression and the role of filler-binders. In Pharmaceutical Dosage Forms: Tablets, 3rd ed; Augsburger, L.L.A., Ed.; Routledge: London, UK; pp. 173–216.
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Uesu, N.Y.; Pineda, E.A.G.; Hechenleitner, A.A.W. Microcrystalline cellulose from soybean husk: Effects of solvent treatments on its properties as acetylsalicylic acid carrier. Int. J. Pharm. 2000, 206, 85–96. [Google Scholar] [CrossRef]
- Ohwoavworhua, F.; Adelakun, T. Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: Characterisation and tableting properties. Indian J. Pharm. Sci. 2010, 72, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achor, M.; Oyeniyi, Y.J.; Yahaya, A. Extraction and characterization of microcrystalline cellulose obtained from the back of the fruit of Lageriana siceraria (water gourd). J. Appl. Pharm. Sci. 2014, 4, 57–60. [Google Scholar]
- Bhattacharya, D.; Germinario, L.T.; Winter, W.T. Isolation, preparation and characterization of cellulose microfibers obtained from bagasse. Carbohydr. Polym. 2008, 73, 371–377. [Google Scholar] [CrossRef]
- Pharmacel® 101. Excipients/Oral-Solid-Dose. Available online: https://dfepharma.com/en/Excipients/Expertise/Oral-Solid-Dose/MCC/Pharmacel-101 (accessed on 29 April 2020).
- SEPPICTM CEOLUSTM. Binders, Disintegrants & Fillers. Available online: https://www.seppic.com/ceolus (accessed on 29 April 2020).
- DuPont Avicel®. Pharmaceutical Products. Available online: https://www.pharma.dupont.com/pharmaceutical-brands/avicelr-for-solid-dose-forms.html (accessed on 29 April 2020).
- Hindi, S.Z.S. Microcrystalline Cellulose: The Inexhaustible Treasure for Pharmaceutical Industry. Nanosci. Nanotechnol. Res. 2017, 4, 17–24. [Google Scholar]
- Benelli, L.; Oliveira, W.P. Fluidized bed coating of inert cores with a lipid-based system loaded with a polyphenol-rich Rosmarinus officinalis extract. Food Bioprod. Process. 2019, 114, 216–226. [Google Scholar] [CrossRef]
- Khan, I.; Apostolou, M.; Bnyan, R.; Houacine, C.; Elhissi, A.; Yousaf, S.S. Paclitaxel-loaded micro or nano transfersome formulation into novel tablets for pulmonary drug delivery via nebulization. Int. J. Pharm. 2019, 575, 118919. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Ghafourian, T.; Nokhodchi, A. Optimising the release rate of naproxen liquid-pellet: A new technology for emerging novel oral dosage form. Drug Deliv. Transl. Res. 2019, 10, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Matos, R.L.; Lu, T.; Leeke, G.; Prosapio, V.; McConville, C.; Ingram, A. Single-step coprecipitation and coating to prepare curcumin formulations by supercritical fluid technology. J. Supercrit. Fluids 2020, 159, 104758. [Google Scholar] [CrossRef]
- Ntoutoume, G.M.N.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorgan. Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef] [Green Version]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Bondancia, T.; Teodoro, K.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Sugarcane bagasse whiskers: Extraction and characterizations. Ind. Crop. Prod. 2011, 33, 63–66. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Dong, S.; Bortner, M.J.; Roman, M. Analysis of the sulfuric acid hydrolysis of wood pulp for cellulose nanocrystal production: A central composite design study. Ind. Crop. Prod. 2016, 93, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Laitinen, O.; Ojala, J.; Sirviö, J.; Liimatainen, H. Sustainable stabilization of oil in water emulsions by cellulose nanocrystals synthesized from deep eutectic solvents. Cellulose 2017, 24, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B. Produção de nanoceluloses integradas ao processo de obtenção de açúcares para etanol 2G a partir de bagaço de cana-de-açúcar. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2018. [Google Scholar]
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The modified nanocrystalline cellulose for hydrophobic drug delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef] [Green Version]
- De Souza, H.J.B.; Botrel, D.A.; Fernandes, R.V.D.B.; Borges, S.V.; Felix, P.H.C.; Viana, L.C.; Lago, A.M.T. Hygroscopic, structural, and thermal properties of essential oil microparticles of sweet orange added with cellulose nanofibrils. J. Food Process. Preserv. 2020, 44, e14365. [Google Scholar] [CrossRef]
- Löbmann, K.; Wohlert, J.; Müllertz, A.; Wågberg, L.; Svagan, A.J.; Hanner, A.J.S. Cellulose Nanopaper and Nanofoam for Patient-Tailored Drug Delivery. Adv. Mater. Interfaces 2017, 4, 1600655. [Google Scholar] [CrossRef]
- Svagan, A.J.; Müllertz, A.; Löbmann, K. Floating solid cellulose nanofibre nanofoams for sustained release of the poorly soluble model drug furosemide. J. Pharm. Pharmacol. 2017, 69, 1477–1484. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.T. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Bhat, A.H.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydr. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.-H.; Lee, J.-S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose nanofibers and other biopolymers for biomedical applications. A review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Min, K.S.; Ji, G.E.; Hwan, J.S.; Mock, L.S.; Jong, S.W.; Sik, K.J. Toxicity Evaluation of Cellulose Nanofibers (Cnfs) for Cosmetic Industry Application. J. Toxicol. Risk Assess. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose 2019, 27, 1301–1314. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, S.; Khalil, H.A.; Haafiz, M.M.; Ishak, Z.M.; Hassan, A.; Sarker, Z. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crop. Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R. Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind. Eng. Chem. Res. 2009, 48, 11211–11219. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Boufi, S.; Pèlach, M.À.; Delgado-Aguilar, M.; Mutjé, P. Evaluation of the fibrillation method on lignocellulosic nanofibers production from eucalyptus sawdust: A comparative study between high-pressure homogenization and grinding. Int. J. Biol. Macromol. 2019, 145, 1199–1207. [Google Scholar] [CrossRef]
- Ferrer, A.; Filpponen, I.; Rodríguez, A.; Laine, J.; Rojas, O. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour. Technol. 2012, 125, 249–255. [Google Scholar] [CrossRef]
- Wang, W.; Mozuch, M.D.; Sabo, R.C.; Kersten, P.; Zhu, J.Y.; Jin, Y. Production of cellulose nanofibrils from bleached eucalyptus fibers by hyperthermostable endoglucanase treatment and subsequent microfluidization. Cellulose 2014, 22, 351–361. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Nanofibrillation of deep eutectic solvent-treated paper and board cellulose pulps. Carbohydr. Polym. 2017, 169, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sain, M.M. Processing of Cellulose Nanofiber-reinforced Composites. J. Reinf. Plast. Compos. 2005, 24, 1259–1268. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Donescu, D.; Spataru, C.I.; Radovici, C.; Trusca, R.; Somoghi, R. Preparation and characterization of PVA composites with cellulose nanofibers obtained by ultrasonication. BioResources 2010, 6, 487–512. [Google Scholar] [CrossRef]
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 2009, 17, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2010, 119, 2652–2660. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2010, 3, 71–85. [Google Scholar] [CrossRef]
- Gamelas, J.A.; Pedrosa, J.; Lourenço, A.F.; Mutjé, P.; González, I.; Chinga-Carrasco, G.; Singh, G.; Ferreira, P.J. On the morphology of cellulose nanofibrils obtained by TEMPO-mediated oxidation and mechanical treatment. Micron 2015, 72, 28–33. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Hussein, M.Z.; Oksman, K. Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 2009, 17, 299–307. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Mills, R.H.; Gardner, D.J.; Bousfield, D. Production and characterization of cellulose nanofibers from wood pulp. J. Adhes. Sci. Technol. 2011, 25, 709–721. [Google Scholar] [CrossRef]
- Pizzi, A.; Belgacem, M.N. Lignocellulosic Fibers and Wood Handbook: Renewable Materials for Today’s Environment; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Valo, H.; Kovalainen, M.; Laaksonen, P.; Häkkinen, M.; Auriola, S.; Peltonen, L.; Linder, M.; Järvinen, K.; Hirvonen, J.T.; Laaksonen, T. Immobilization of protein-coated drug nanoparticles in nanofibrillar cellulose matrices—Enhanced stability and release. J. Control. Release 2011, 156, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.; Serimaa, R.; Kuga, S.; Hirvonen, J.T.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2019, 275, 102048. [Google Scholar] [CrossRef]
- Santus, G.; Baker, R.W. Controlled Release of Pharmaceuticals. In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: New York, NY, USA, 2003; pp. 791–803. [Google Scholar]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.T.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Bannow, J.; Benjamins, J.-W.; Wohlert, J.; Löbmann, K.; Svagan, A. Solid nanofoams based on cellulose nanofibers and indomethacin—The effect of processing parameters and drug content on material structure. Int. J. Pharm. 2017, 526, 291–299. [Google Scholar] [CrossRef]
- Bera, S.; Dutta, D. Encapsulation and release of a bacterial carotenoid from hydrogel matrix: Characterization, kinetics and antioxidant study. Eng. Life Sci. 2017, 17, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Gharakhloo, M.; Sadjadi, S.; Rezaeetabar, M.; Askari, F.; Rahimi, A. Cyclodextrin-Based Nanosponges for Improving Solubility and Sustainable Release of Curcumin. ChemistrySelect 2020, 5, 1734–1738. [Google Scholar] [CrossRef]
- Samadi, N.; Azar, P.A.; Husain, S.W.; Maibach, H.I.; Nafisi, S. Experimental design in formulation optimization of vitamin K1 oxide-loaded nanoliposomes for skin delivery. Int. J. Pharm. 2020, 579, 119136. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhu, Q.; Diao, C.; Wang, J.; Wu, Z.; Wang, H. Enhanced physicochemical stability of lutein-enriched emulsions by polyphenol-protein-polysaccharide conjugates and fat-soluble antioxidant. Food Hydrocoll. 2019, 101, 105447. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core–Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic-co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Ramanathan, M.; Sankar, V. Preparation, characterization and in vitro cytotoxicity assay of curcumin loaded solid lipid nanoparticle in IMR32 neuroblastoma cell line. Pak. J. Pharm. Sci. 2014, 27. [Google Scholar]
- Salem, A.; Ramadan, A.R.; Shoeib, T. Entrapment of β-carotene and zinc in whey protein nanoparticles using the pH cycle method: Evidence of sustained release delivery in intestinal and gastric fluids. Food Biosci. 2018, 26, 161–168. [Google Scholar] [CrossRef]
- Sáiz-Abajo, M.-J.; González-Ferrero, C.; Moreno-Ruiz, A.; Hualde, A.R.; González-Navarro, C.J. Thermal protection of β-carotene in re-assembled casein micelles during different processing technologies applied in food industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.A.; Sbihi, H.M.; Al-Resayes, S.I.; Malmiri, H.J.; Tan, C.P. Preparation of Astaxanthin Nanodispersions Using Gelatin-Based Stabilizer Systems. Molecules 2014, 19, 14257–14265. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.-X.; Zhang, N.; Tang, C.-H. Soy protein isolate as a nanocarrier for enhanced water dispersibility, stability and bioaccessibility of β-carotene. J. Sci. Food Agric. 2016, 97, 2230–2237. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Chen, L. Elaboration and characterization of barley protein nanoparticles as an oral delivery system for lipophilic bioactive compounds. Food Funct. 2013, 5, 92–101. [Google Scholar] [CrossRef]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-Based Structures for Food Applications: From Macro to Nanoscale. Front. Sustain. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Sharma, P.K.S.P.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel Encapsulation of Lycopene in Niosomes and Assessment of its Anticancer Activity. J. Bioequiv. Bioavailab. 2016, 8. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of nanostructured lipid carriers for the encapsulation and controlled release of vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.; Fonseca, L.P. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind. Crop. Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Z.; Li, D.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Sunkaria, A.; Singhal, N.; Sandhir, R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.S.; Syed, I.; Sivabalan, S.; Sarkar, P. Nanoencapsulation strategies for lipid-soluble vitamins. Chem. Pap. 2019, 73, 1–16. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery. Trends Food Sci. Technol. 2019, 88, 397–415. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L. Polymeric materials as carriers for drug delivery systems. In Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 321–348. [Google Scholar]
- Akhlaghi, S.P.; Berry, R.C.; Tam, K.C. Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 2013, 20, 1747–1764. [Google Scholar] [CrossRef]
- Merck. Portugal. Available online: https://www.sigmaaldrich.com (accessed on 1 September 2021).
- ChemSpider. Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 20 September 2021).
- The United States Pharmacopeia. The United States Pharmacopeia (USP) (1995). The National Formulary, 23rd ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 1995; pp. 131–145. [Google Scholar]
- De Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled release of carvacrol and curcumin: Bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Omidi, S.; Pirhayati, M.; Kakanejadifard, A. Co-delivery of doxorubicin and curcumin by a pH-sensitive, injectable, and in situ hydrogel composed of chitosan, graphene, and cellulose nanowhisker. Carbohydr. Polym. 2019, 231, 115745. [Google Scholar] [CrossRef]
- Cao, Y. Applications of cellulose nanomaterials in pharmaceutical science and pharmacology. Express Polym. Lett. 2018, 12, 768–780. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, R.; Zhang, Y.; Xue, W.; Cheng, B.; Zhang, Y. Controlled Dual Delivery of Angiogenin and Curcumin by Electrospun Nanofibers for Skin Regeneration. Tissue Eng. Part A 2017, 23, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555. [Google Scholar] [CrossRef] [PubMed]
- Svagan, A.J.; Musyanovych, A.; Kappl, M.; Bernhardt, M.; Glasser, G.; Wohnhaas, C.; Berglund, L.A.; Risbo, J.; Landfester, K. Cellulose nanofiber/nanocrystal reinforced capsules: A fast and facile approach toward assembly of liquid-core capsules with high mechanical stability. Biomacromolecules 2014, 15, 1852–1859. [Google Scholar] [CrossRef]
- Svagan, A.; Koch, C.B.; Hedenqvist, M.; Nilsson, F.; Glasser, G.; Baluschev, S.; Andersen, M. Liquid-core nanocellulose-shell capsules with tunable oxygen permeability. Carbohydr. Polym. 2016, 136, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kotkoskie, L.A.; Butt, M.T.; Selinger, E.; Freeman, C.; Weiner, M.L. Qualitative investigation of uptake of fine particle size microcrystalline cellulose following oral administration in rats. J. Anat. 1996, 189, 531–535. [Google Scholar]
- Additives, FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- EFSA. Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA J. 2018, 16, e05047. [Google Scholar] [CrossRef] [Green Version]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; Clift, M.J.D. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Ni, H.; Zeng, S.; Wu, J.; Cheng, X.; Luo, T.; Wang, W.; Zeng, W.; Chen, Y. Cellulose nanowhiskers: Preparation, characterization and cytotoxicity evaluation. In Bio-Medical Materials and Engineering; IOS Press: Amsterdam, The Netherlands, 2012; pp. 121–127. [Google Scholar]
- Hanif, Z.; Ahmed, F.R.; Shin, S.W.; Kim, Y.-K.; Um, S.H. Size- and dose-dependent toxicity of cellulose nanocrystals (CNC) on human fibroblasts and colon adenocarcinoma. Colloids Surf. B Biointerfaces 2014, 119, 162–165. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- DeLoid, G.M.; Cao, X.; Molina, R.M.; Silva, D.I.; Bhattacharya, K.; Ng, K.W.; Loo, S.C.J.; Brain, J.D.; Demokritou, P. Toxicological effects of ingested nanocellulose in in vitro intestinal epithelium and in vivo rat models. Environ. Sci. Nano 2019, 6, 2105–2115. [Google Scholar] [CrossRef]
- Burchett, J.H. Evaluating the Cytotoxic Effects of Cellulose Nanocrystals (CNCs) Using Autobioluminescent Yeast and Human Cells; The University of Tennessee: Knoxville, TN, USA, 2016. [Google Scholar]
- Pereira, M.M.; Raposo, N.R.B.; Brayner, R.; Teixeira, E.M.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.S.; Mattoso, L.H.C.; Brandão, H. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef]
- Mahmoud, K.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J. Effect of Surface Charge on the Cellular Uptake and Cytotoxicity of Fluorescent Labeled Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Ålander, E.; Lindström, T.; Mihranyan, A.; Strømme, M.; Ferraz, N. Surface chemistry of nanocellulose fibers directs monocyte/macrophage response. Biomacromolecules 2015, 16, 2787–2795. [Google Scholar] [CrossRef]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J. Probing inhibitory effects of nanocrystalline cellulose: Inhibition versus surface charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Sim, G.; Alam, N.; Tufenkji, N.; Van De Ven, T.G.M. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 2015, 7, 16647–16657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebert, T.; Kostag, M.; Wotschadlo, J.; Heinze, T. Stable Cellulose Nanospheres for Cellular Uptake. Macromol. Biosci. 2011, 11, 1387–1392. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132. [Google Scholar] [CrossRef]
- Clift, M.; Foster, J.; Vanhecke, D.; Studer, D.; Wick, P.; Gehr, P.; Rothen-Rutishauser, B.; Weder, C. Investigating the Interaction of Cellulose Nanofibers Derived from Cotton with a Sophisticated 3D Human Lung Cell Coculture. Biomacromolecules 2011, 12, 3666–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endes, C.; Mueller, S.; Kinnear, C.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Weder, C.; Clift, M.J.D.; Rothen-Rutishauser, B. Fate of Cellulose Nanocrystal Aerosols Deposited on the Lung Cell Surface In Vitro. Biomacromolecules 2015, 16, 1267–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zheng, G.-J.; Guo, Y.-T.; Zhou, L.; Du, J.; He, H. Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization. Asian Pac. J. Trop. Med. 2014, 7, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Li, Y.; Yang, B.; Xiao, D.; Zhang, S.; Rajulu, A.V.; Kondo, T.; Zhang, L.; Zhou, J. Effect of microcrystal cellulose and cellulose whisker on biocompatibility of cellulose-based electrospun scaffolds. Cellulose 2013, 20, 1911–1923. [Google Scholar] [CrossRef]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Menz, J.; Schubert, T.; Thielemans, W. Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 2011, 82, 1387–1392. [Google Scholar] [CrossRef]
- Carrière, F. Impact of gastrointestinal lipolysis on oral lipid-based formulations and bioavailability of lipophilic drugs. Biochimie 2016, 125, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.A.O.; Mercadante, A.Z. The bioaccessibility of carotenoids impacts the design of functional foods. Curr. Opin. Food Sci. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.D.S. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Kamiloglu, S.; Capanoglu, E. models for studying polyphenols and carotenoids digestion, bioaccessibility and colonic fermentation. In Food Chemistry, Function and Analysis; Williamson, G., Marangoni, A.G., Bonwick, G.A., Birch, C.S., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 201–219. [Google Scholar]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [Green Version]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are lutein, lycopene, and β-carotene lost through the digestive process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact ofin vitrodigestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; André, C.M.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chem. 2015, 197, 325–332. [Google Scholar] [CrossRef]
- Garcea, G.; Jones, D.; Singh, R.; Dennison, A.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.M.; Ho, Y.-F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.-R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Prosapio, V.; Reverchon, E.; De Marco, I. Incorporation of liposoluble vitamins within PVP microparticles using supercritical antisolvent precipitation. J. CO2 Util. 2017, 19, 230–237. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2018, 89, 80–89. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Wang, H.; Roman, M. Formation and Properties of Chitosan−Cellulose Nanocrystal Polyelectrolyte−Macroion Complexes for Drug Delivery Applications. Biomacromolecules 2011, 12, 1585–1593. [Google Scholar] [CrossRef]
- Sheikhi, A.; Hayashi, J.; Eichenbaum, J.; Gutin, M.; Kuntjoro, N.; Khorsandi, D.; Khademhosseini, A. Recent advances in nanoengineering cellulose for cargo delivery. J. Control. Release 2018, 294, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Jinno, J.-I.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, D.; Kagan, D. Bioavailability of a sustained release formulation of curcumin. Integr. Med. 2014, 13, 24–30. [Google Scholar]
- Mantas, A.; Labbe, V.; Loryan, I.; Mihranyan, A. Amorphisation of Free Acid Ibuprofen and Other Profens in Mixtures with Nanocellulose: Dry Powder Formulation Strategy for Enhanced Solubility. Pharmaceutics 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, P.; Rawal, S.; Patel, B.; Patel, M.M. Oral Delivery of Anticancer Agents Using Nanoparticulate Drug Delivery System. Curr. Drug Metab. 2020, 20, 1132–1140. [Google Scholar] [CrossRef]
- Wagner, A.M.; Gran, M.P.; Peppas, N.A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm. Sin. B 2018, 8, 147–164. [Google Scholar] [CrossRef]
- Koshani, R.; Madadlou, A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci. Technol. 2018, 71, 268–273. [Google Scholar] [CrossRef]
- Jiang, F.; Esker, A.; Roman, M. Acid-catalyzed and solvolytic desulfation of H2SO4-hydrolyzed cellulose nanocrystals. Langmuir 2010, 26, 17919–17925. [Google Scholar] [CrossRef]
- Crater, J.S.; Carrier, R.L. Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport. Macromol. Biosci. 2010, 10, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Roblegg, E.; Fröhlich, E.; Meindl, C.; Teubl, B.; Zaversky, M.; Zimmer, A. Evaluation of a physiologicalin vitrosystem to study the transport of nanoparticles through the buccal mucosa. Nanotoxicology 2011, 6, 399–413. [Google Scholar] [CrossRef]
- Liu, L.; Kerr, W.L.; Kong, F. Characterization of lipid emulsions during in vitro digestion in the presence of three types of nanocellulose. J. Colloid Interface Sci. 2019, 545, 317–329. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kong, F. In vitro investigation of the influence of nano-cellulose on starch and milk digestion and mineral adsorption. Int. J. Biol. Macromol. 2019, 137, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Shatkin, J.A.; Kong, F. Evaluating mucoadhesion properties of three types of nanocellulose in the gastrointestinal tract in vitro and ex vivo. Carbohydr. Polym. 2019, 210, 157–166. [Google Scholar] [CrossRef] [PubMed]







| Cellulose Source | NC Material | Isolation Method(s) | Isolation Conditions | Surface Chemistry | Material Properties | Product Yield | Reference |
|---|---|---|---|---|---|---|---|
| Bleached commercial softwood | CNC | AH + S | AH: H2SO4 64% w/w; ratio: 8.75 mL/g; 45 °C/25 min S: 10 min, 60% power | Sulfate groups | CI: >90% Size: ca. 10 vs. 500 nm Film Morphology: layered, unidirectional ZP: −55 mV | - | [18] |
| Kenaf bast | CNC | AH | AH: H2SO4 64% w/w; 45 °C/40 min | Sulfate groups | CI: 71.9% Size: 4–20 vs. 50–200 nm Aspect ratio: 13.4 Morphology: Rod-shaped TG: 180 °C, 230 °C | 41% | [39] |
| Empty fruit bunch | CNC | AH + S | AH: H2SO4 58% w/w; 45 °C/45 min S: 10 min, 60% power | Sulfate groups | CI: 77.6% Size: 13–30 vs. 150–360 nm Aspect ratio: 27 TG: 200 °C Contact angle: 45° | - | [5] |
| Cotton | CNC | AH | AH: H2SO4 64% w/w; ratio: 20 mL/g 45 °C/45 min Water dialysis | Sulfate groups | Size: ca. 140 nm lenght ZP: −55 mV | 25% | [95] |
| Date palm stalks | CNC | AH | AH: H2SO4 64% w/w; ratio 20 mL/g 45 °C/45 min S: 6 min, 200 W | Sulfate groups | CI: 78% Size: 5–7 vs. 86–237 nm ZP: −53.8 mV | [28] | |
| Micro-crystalline cellulose | CNC | AH | AH: H2SO4 64% w/w; ratio: 12 mL/g; 45 °C/30 min | Sulfate groups | CI: 70.2% Size: ca. 50 nm wide Morphology: spheroid | - | [114] |
| Bleached kraft pulp (eucalyptus) | CNF | Defibrillation | 1500 rpm; 5 passes | Hydroxyl groups | Size: 17–40 nm vs. 2–12 µm Morphology: twisted and elongated fibers; non-individual network | - | [115] |
| Bleached sulfite pulp (spruce) | CNF | HPH | 1650 bar (chambers 400/100µm); 2 passes | Hydroxyl groups | CI: 48% Size: 4–6 nm vs. several µm DS: 0.44 mmol/g | - | [116] |
| Bleached sulfite pulp | CNF | HPH | 1650 bar (chambers 400/100 μm); 2 passes | Hydroxyl groups | Size: 3–5 nm vs. several μm | - | [117] |
| Encapsulating Material(s) | Active Ingredient(s) | Encapsulation Method | Final Structure | Encapsulation Results | Release Results | Application | Reference |
|---|---|---|---|---|---|---|---|
| MCC treated with ethanol, acetone, chloroform, or ethyl ether | Acetylsalicylic acid | Solvent Evaporation | Microparticles | Size: ca. 100 µm Morphology: elongated TG: 160 °C | Commercial MCC; Solvent: Buffer pH 4.5 Acetone: 90%/30 min Other solvents: 20–30%/30 min Commercial MCC; Solvent: pure water Acetone: 100%/2 h Ether: 45%/8 h Other solvents: 80%/8 h Prepared MCC; Solvent: Buffer pH 4.5 15–30%/30 min Prepared MCC; Solvent: pure water Chloroform and no solvent: 80%/8 h Other solvents: 40–55%/8 h | Pharma | [83] |
| MCC + Lipid system (Poloxamer 407, stearic acid) + Maltodextrin DE10 + Gum arabic or Whey protein | Rosmarinus officinalis extract, including Carnosic acid and Carnosol | Fluidized Bed Spray Coating | Microparticles | EE = 80–90% Coating efficiency = 65–80% Morphol.: Spherical Size: 600–800 µm Excellent flow properties | - | Food, Cosmetic, Pharma | [91] |
| MCC + SPC + Cholesterol + Surfactant (Span 80, Span 20 or Tween 80) | Paclitaxel | Slurry Method | Protransfersome | EE = 92–98% Morphol.: oblong ZP: −2.52 mV | - | Pharma | [92] |
| MCC + Tween 80 + Hydrophilic fumed silica + Sodium starch glycolate | Naproxen | Extrusion + Spheronisation | Liqui-pellet | Size: ca. 1 mm Morphol.: spheroid Excellent flow properties | Solvent: HCl buffer (pH 1.2), PBS (pH 7.4) pH 7.4: from 80%/2 h to 100%/15 min (different formulations) pH 1.2: 5–20% released in 2 h | Pharma | [93] |
| MCC + PVP | Curcumin | Supercritical Anti-solvent + Fluidized Bed | Microparticles | Size: ca. 140 µm Morphol.: spherical Excellent flow properties | Solvent: 0.25 % w/v SDS Without PVP: 50%/1 h With PVP 100%/5 min | Pharma | [94] |
| Encapsulating Material(s) | Active Ingredient(s) | Method | Final Structure | Encapsulation Results | Release Results | Application | Reference |
|---|---|---|---|---|---|---|---|
| CNC modified with CTBA | Docetaxel, Paclitaxel and Etoposide | Incubation | Nanocomplexes | EE (DTX, PTX) = 90% EE (ETOP) = 48% | Solvent: PBS (pH 7.4) Rapid release: 20%/1 h DTX: 59%/2 d PTX: 44%/2 d ETOP: 75%/2 d | Pharma | [18] |
| CNC + Chitosan | Curcumin | Layer-by-Layer assembly | Multilayer (n = 10) films Multilayer (n = 5) microcapsules | LC: 1.74 μg/cm Morphology: porous, nanofibrous | Solvent: PBS (pH 7.4) Rapid release: 35%/1 h 65% released/8 h Release kinetics: Korsmeyer model 0.22 release exponent | Pharma | [15] |
| CNC + Cationic cyclodextrins | Curcumin | Electrostatic coupling + Incubation | Nanocomplexes | LC = 8–10% ZP: −30 mM | Solvent: H2O/CHCl3 Rapid release: 15%/1 h 20–25%/8 h Enhanced antiproliferative effect on colorectal and prostatic cancer cell lines | Pharma | [95] |
| CNC + Chitosan | Curcumin | Swelling equilibrium | Hydrogel | EE: 41% Morphology: interconnected, porous Swelling ratio: 438% | Solvent: simulated gastric medium Prolonged release phase at 2.5 h (0.70 mg/L) | Pharma | [179] |
| CNC + PLGA | Curcumin | Electrospinning | Composite nanofibers | Size: 100–200 nm wide | Solvent: PBS (pH 7.4) 74%/1 d; 90%/6 d Bioactivity of Cur preserved Excellent biocompatibility | Pharma | [183] |
| CNC + Collagen as scaffold Gelatin as carrier | Curcumin | Emulsion solvent evaporation + Freeze-Drying | Scaffolds containing curcumin-loaded microspheres | Morphology: interconnected, porous Pore size: 80–110 μm Porosity: 90% | Solvent: DTM solution 35%/1 d 100%/10 d | Pharma | [184] |
| CNC modified with CTBA | Curcumin | Incubation | Nanocomplexes | EE (unmodified CNC) = 27% EE (CTBA-CNC) = 80–96% | - | Pharma | [39] |
| TEMPO-oxidized CNC (TOCNC) + HPβCD + PEG200 | Curcumin Carvacrol | Casting + Impregnation | Films | Loading of carvacrol and curcumin increased compared with virgin TOCNC | Solvent: distilled water Curcumin: 95–100%/2 h Carvacrol: 90–100%/2 h TOCNC/HPβCD loading carvacrol exhibited excellent antibacterial activities | Food packaging | [178] |
| CNC modified with TA and DA | Curcumin | Incubation | Nanocomplexes | EE (unmodified CNC) = 8–54% EE (TA-DA-CNC) = 95–99% | - | Pharma | [5] |
| Aminated-CNC + Chitosan + Aminated-Graphene + synthetic dialdehyde | Curcumin | Schiff base reaction | Hydrogel | Morphology: cross-linked, porous Swelling ratio: 6985% | Solvent: PBS (pH 7.4 and 5.4) pH 7.4: 25%/12 h pH 5.4: 55%/12 h Fast gelation in rat’s skin by subcutaneous injections Antibacterial activity against gram-positive bacteria | Pharma | [180] |
| CNC modified with CTBA | Luteolin Luteoloside | Incubation | Nanocomplexes | LC (luteolin) = 12.9 mg/g LC (luteoloside) = 56.9 mg/g ZP: ca. −30 mV | Solvent: PBS (pH 7.4 and 6.4) Luteolin pH 7.4: 57%/24 h pH 6.4: 44%/24 h Luteoloside pH 7.4: 72%/24 h pH 6.4: 57%/24 h | - | [114] |
| Magnetic CNC + Alginate | Ibuprofen | Co-precipitation + Extrusion into a CaCl2 gelation bath | Hydrogel beads | EE = 38% LC = 3.2% Size: 2.3–2.4 nm (wet), 1.9–2.0 mm (freeze dried) Morphology: ellipsoidal, wrinkled Swelling ratio: 1878–2477% | Solvent: PBS (pH 7.4) Rapid release: 45–60%/30 min 100%/5–6 h | Pharma | [29] |
| CNC, TOCNC or ACNC + Chitosan + TPP | Ketoprofen | Ionic gelation | Nanoparticles | EE = 73–79% ZP: ca. 30 mV Size: 195–235 nm Morphology: spherical PDI: 0.1–0.2 | Solvent: PBS (pH 7.4) Rapid release: 20–50%/2 h CNC: 41–46%/6 h TOCNC: 58–62%/6 h ACNC: 60–64%/6 h | Pharma | [28] |
| Encapsulating material(s) | Active ingredient(s) | Encapsulation Method | Final Structure | Encapsulation results | Release results | Application | Reference |
|---|---|---|---|---|---|---|---|
| CNF/CNC as water phase Spin-probe, IPDI, and dibutyltin dilaurate as oil phase TOCNF as matrix | Hexadecane | Direct mini-emulsion polymerization + Filtration through a hydrophobic membrane | Microcapsules containing several primary capsules in a CNF matrix | Size: Primary capsule = 1–2 μm Aggregate capsule = 6–11 μm Oxygen uptake rate was reduced for both capsules | - | Food, Pharma | [186] |
| CNF + Gum Arabic | Sweet orange essential oil | Sonication + Spray drying | Microparticles | LC: 17.0% Morphology: spherical, wrinkled TG: 323 °C | - | Food | [115] |
| CNF | Ibuprofen | Sonication + Spray drying | Microparticles | LC: 1.7% Morphology: fibrous, spheroid Size: ca. 5 µm | Solvent: PBS (pH 7.4) Slow-release rate over 2 months | Pharma | [118] |
| CNF | Furosemide | Casting + Drying | Nanofoams | LC: 21%, 50% Size: 0.4–0.8 mm thick Density: ca. 0.035 g/cm3 Porosity: 98% | Solvent: simulated gastric fluid (pH 1.6) Rapid release of ca.25%/2 h 50% wt foam: 45%/24 h 21% wt foam: 65%/24 h | Pharma | [150] |
| HFBI as coating CNF + Trehalose as matrix | Itraconazole | Anti-solvent precipitation + Freeze-drying | Immobilized particles in CNF matrices | Particle size: ca.100 nm CNFs played a critical effect on the stabilization of the particles (storage for more than ten months) | Solvent: NaCl/HCl solution (pH 1.2) Rapid release of ca. 60%/10 min Before storage: 90%/90 min After 12 weeks storage: 75%/90 min | Pharma | [144] |
| CNF | Itraconazole | Sonication + Drying | Films | EE: >80% LC: 17–40% | Solvent: NaCl/HCl solution (pH 1.2) 55–90%/80 d Zero-order release kinetics | Pharma | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, F.; Pereira, C.F.; Ribeiro, A.B.; Freixo, R.; Costa, E.; E. Pintado, M.; Fernandes, J.C.; Ramos, Ó.L. Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds. Nanomaterials 2021, 11, 2593. https://doi.org/10.3390/nano11102593
Casanova F, Pereira CF, Ribeiro AB, Freixo R, Costa E, E. Pintado M, Fernandes JC, Ramos ÓL. Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds. Nanomaterials. 2021; 11(10):2593. https://doi.org/10.3390/nano11102593
Chicago/Turabian StyleCasanova, Francisca, Carla F. Pereira, Alessandra B. Ribeiro, Ricardo Freixo, Eduardo Costa, Manuela E. Pintado, João C. Fernandes, and Óscar L. Ramos. 2021. "Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds" Nanomaterials 11, no. 10: 2593. https://doi.org/10.3390/nano11102593
APA StyleCasanova, F., Pereira, C. F., Ribeiro, A. B., Freixo, R., Costa, E., E. Pintado, M., Fernandes, J. C., & Ramos, Ó. L. (2021). Novel Micro- and Nanocellulose-Based Delivery Systems for Liposoluble Compounds. Nanomaterials, 11(10), 2593. https://doi.org/10.3390/nano11102593