Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
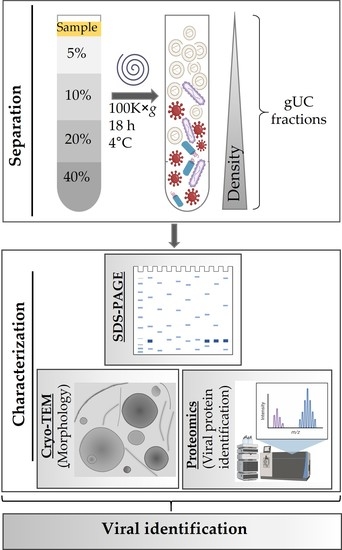
2.2. Isolation of Tomato Nanovesicles by Differential Centrifugation
2.3. Fractionation of Tomato Nanovesicles by Density Gradient Ultracentrifugation and Density Determination
2.4. Size-Exclusion Chromatography of Tomato-Derived Nanovesicles
2.5. Determination of Physical and Molecular Characteristics of Tomato-Derived NVs
2.6. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.7. Scanning Electron Microscopy (SEM)
2.8. LC–ESI–MS/MS Analysis
2.9. Bioinformatics
3. Results
3.1. Isolation and Characterization of Tomato-Derived Nanovesicles
3.2. Cryo-TEM Analysis Shows Viral Contamination in some Tomato Nanovesicles Samples
3.3. Proteomics Reveals the Identity of Viral Particles-Related Proteins in Tomato-Derived Nanovesicles
3.3.1. Sample 1 Contains Tomato Vesicles and Three Different Viral Particles
3.3.2. Sample 2 Contains Tomato Vesicles in the Low-Density and Viral Particles in the High-Density Sucrose Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. Peer J. 2018, 6, e5186. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Woith, E.; Melzig, M.F. Extracellular vesicles from fresh and dried plants—Simultaneous purification and visualization using gel electrophoresis. Int. J. Mol. Sci. 2019, 20, 357. [Google Scholar] [CrossRef] [Green Version]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, C.A.; Walker, G.P.; Ullman, D.E. Infection with a plant virus modifies vector feeding behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 9350–9355. [Google Scholar] [CrossRef] [Green Version]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 1–18. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; Zhang, H. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2016, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L.; Pocsfalvi, G. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of Tomato-Derived Nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E.L. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Cameron, G.T.; Geana, M.V. Symposium: Relative Bioactivity of Functional Foods and Related Dietary Supplements Functional Foods: Delivering Information to the Oncology Nurse 1, 2. J. Nutr. 2005, 1, 1253–1255. [Google Scholar] [CrossRef] [Green Version]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.N.; Taheri, S.; Othman, R.Y.; Teo, C.H. Viral disease of tomato crops (Solanum lycopesicum L.): An overview. J. Plant Dis. Prot. 2020, 127, 725–739. [Google Scholar] [CrossRef]
- Caciagli, P. Vegetable Viruses. Encycl. Virol. 2008, 282–290. [Google Scholar] [CrossRef]
- Ambrós, S.; Martínez, F.; Ivars, P.; Hernández, C.; de la Iglesia, F.; Elena, S.F. Molecular and biological characterization of an isolate of Tomato mottle mosaic virus (ToMMV) infecting tomato and other experimental hosts in eastern Spain. Eur. J. Plant Pathol. 2017, 149, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Turina, M.; Geraats, B.P.J.; Ciuffo, M. First report of Tomato mottle mosaic virus in tomato crops in Israel. New Dis. Rep. 2016, 33, 1. [Google Scholar] [CrossRef] [Green Version]
- Oladokun, J.O.; Halabi, M.H.; Barua, P.; Nath, P.D. Tomato brown rugose fruit disease: Current distribution, knowledge and future prospects. Plant Pathol. 2019, 68, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- Wilstermann, A.; Ziebell, H. Tomato brown rugose fruit virus (ToBRFV). JKI Data Sheets Plant Dis. Diagn. 2019, 1, ISSN 2191-1398. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kwon, S.Y.; Kim, S.T. An insight into the tomato spotted wilt virus (TSWV), tomato and thrips interaction. Plant Biotechnol. Rep. 2018, 12, 157–163. [Google Scholar] [CrossRef]
- Turina, M.; Kormelink, R.; Resende, R.O. Resistance to Tospoviruses in Vegetable Crops: Epidemiological and Molecular Aspects. Annu. Rev. Phytopathol. 2016, 54, 347–371. [Google Scholar] [CrossRef]
- Abadkhah, M.; Koolivand, D.; Eini, O. A new distinct clade for Iranian Tomato spotted wilt virus isolates based on the polymerase, nucleocapsid, and non-structural genes. Plant Pathol. J. 2018, 34, 514–531. [Google Scholar] [CrossRef]
- Sether, D.M.; DeAngelis, J.D. Tomato Spotted Wilt Virus Host List and Bibliography; Special Report 888; Oregon State University: Corvallis, OR, USA, 1992. [Google Scholar]
- Mandal, B.; Jain, R.K. Can plant virus infect human being? Indian J. Virol. 2010, 21, 92–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balique, F.; Lecoq, H.; Raoult, D.; Colson, P. Can plant viruses cross the kingdom border and be pathogenic to humans? Viruses 2015, 7, 2074–2098. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Yoon, S.J.; Park, Y.J.; Kim, S.Y.; Ryu, C.M. Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 2020, 22, 2485–2495. [Google Scholar] [CrossRef]
- De Toledo Martins, S.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Hoen, E.N.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [Green Version]
- Pocsfalvi, G.; Mammadova, R.; Ramos Juarez, A.P.; Bokka, R.; Trepiccione, F.; Capasso, G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press. Res. 2020, 45, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Mcnamara, R.P.; Dittmer, D.P. Modern Techniques for the Isolation of Extracellular Vesicles and Viruses. J. Neuroimmune Pharmacol. 2020, 15, 459–472. [Google Scholar] [CrossRef]
- Hun, Y.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar]
- Balke, I.; Zeltins, A. Recent advances in the use of plant virus-like particles as vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://biorender.com/ (accessed on 5 June 2021).
- Lešer, V.; Drobne, D.; Pipan, Ž.; Milani, M.; Tatti, F. Comparison of different preparation methods of biological samples for FIB milling and SEM investigation. J. Microsc. 2009, 233, 309–319. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havliš, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef]
- Bioinformatics Made Easy (Version 1.4.12). Available online: https://www.biobam.com/omicsbox (accessed on 3 March 2019).
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Kralj-Iglič, V.; Pocsfalvi, G.; Mesarec, L.; Šuštar, V.; Hägerstrand, H.; Iglič, A. Minimizing isotropic and deviatoric membrane energy—A unifying formation mechanism of different cellular membrane nanovesicle types. PLoS ONE 2020, 15, e0244796. [Google Scholar] [CrossRef]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato virus Y: A major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Kežar, A.; Kavčič, L.; Polák, M.; Nováček, J.; Gutiérrez-Aguirre, I.; Žnidarič, M.T.; Coll, A.; Stare, K.; Gruden, K.; Ravnikar, M.; et al. Structural basis for the multitasking nature of the potato virus Y coat protein. Sci. Adv. 2019, 5, eaaw3808. [Google Scholar] [CrossRef] [Green Version]
- Fukuhara, T.; Tabara, M.; Koiwa, H.; Takahashi, H. Effect of asymptomatic infection with southern tomato virus on tomato plants. Arch. Virol. 2020, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; Zhang, H.G. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.W.; Uchiyama, K. Uptake of micrornas from exosome-like nanovesicles of edible plant juice by rat enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, H.M. CRC Handbook of Viruses: Mass-Molecular Weight Value and Related Properties; CRC Press LLC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Hartmann, M.; Kim, D.; Bernsdorff, F.; Ajami-Rashidi, Z.; Scholten, N.; Schreiber, S.; Zeier, T.; Schuck, S.; Reichel-Deland, V.; Zeier, J. Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity. Plant Physiol. 2017, 174, 124–153. [Google Scholar] [CrossRef] [Green Version]








| No. | Accession | Description UniProt | PLGS Score | Peptides | Coverage (%) | Description OmicsBOX (Protein Blast) | Sim Mean |
|---|---|---|---|---|---|---|---|
| 1 | A0A3Q7IXE6 | Uncharacterized protein | 14,872 | 47 | 79 | patellin-3-like | 94.26 |
| 2 | Q84XW6 | V-ATPase 69 kDa subunit | 29,199 | 39 | 76 | V-type proton ATPase catalytic subunit A | 98.78 |
| 3 | P38416 | Linoleate 9S-lipoxygenase B | 9129 | 32 | 44 | putative linoleate 9S-lipoxygenase 5 | 90.26 |
| 4 | A0A3Q7ENA3 | Lipoxygenase | 9055 | 31 | 43 | putative linoleate 9S-lipoxygenase 5 | 90.1 |
| 5 | Q84XV7 | V-ATPase 69 kDa subunit | 19,723 | 29 | 51 | V-type proton ATPase catalytic subunit A | 98.78 |
| 6 | Q9XEX8 | Remorin 1 | 10,015 | 22 | 51 | remorin | 86.7 |
| 7 | A0A3Q7FE06 | V-type proton ATPase subunit a | 72,645 | 22 | 35 | V-type proton ATPase subunit a3 | 95.26 |
| 8 | Q9SPD5 | Plasma membrane ATPase | 6306 | 22 | 28 | plasma membrane atpase 1 | 99.29 |
| 9 | A0A3Q7IIS5 | Vacuolar proton pump subunit B | 24,212 | 21 | 62 | V-type proton ATPase subunit B2 | 98.52 |
| 10 | A0A3Q7IZ03 | Uncharacterized protein | 15,503 | 21 | 45 | heat shock cognate 70 kDa protein 2-like | 98.17 |
| 11 | A0A3Q7FX57 | Uncharacterized protein | 15,788 | 20 | 42 | heat shock cognate 70 kDa protein 2-like | 99.12 |
| 12 | A0A3Q7INZ6 | Uncharacterized protein | 44,097 | 18 | 48 | actin-7 | 99.19 |
| 13 | A0A3Q7GJM0 | Phosphoinositide phospholipase | 5957 | 18 | 38 | phosphoinositide phospholipase C 2-like | 95.35 |
| 14 | A0A3Q7FJJ3 | Uncharacterized protein | 43,162 | 17 | 51 | actin-7 | 99.87 |
| 15 | A0A3Q7FRW6 | PHB domain-containing protein | 12,933 | 17 | 50 | hypersensitive-induced reaction 1 protein | 99.01 |
| 16 | A0A3Q7EZ16 | 14_3_3 domain-containing protein | 8764 | 17 | 58 | 14-3-3 protein 4 | 97.82 |
| 17 | A0A3Q7IYI9 | Uncharacterized protein | 14,977 | 16 | 35 | heat shock cognate 70 kDa protein | 98.88 |
| 18 | A0A3Q7FV11 | H(+)-exporting diphosphatase | 7652.922 | 16 | 13 | pyrophosphate-energized vacuolar membrane proton pump-like | 98.42 |
| 19 | A0A3Q7I767 | Fe2OG dioxygenase domain-containing protein | 7169.406 | 16 | 37 | 1-aminocyclopropane-1-carboxylate oxidase homolog | 83.31 |
| 20 | A0A3Q7HFP1 | Glycerophosphodiester phosphodiesterase | 6081.231 | 16 | 22 | glycerophosphodiester phosphodiesterase GDPDL4 | 88.91 |
| Name of the Virus | Genus of the Virus | Viral Characteristics | Sample | gUC Fraction(s) * | Name of Viral Protein(s) Identified | UniProt Accession No. | Coverage % of Viral Protein(s) Identified |
|---|---|---|---|---|---|---|---|
| Tomato brown rugose fruit virus (ToBRFV) | Tobamovirus | Single-stranded RNA rod-shaped particles of 300 nm in length and 17 nm in diameter [31] | S1 | 4 7 9 | Capsid protein | A0A0S2SZX3 | 55.3 54.7 55.4 |
| Tomato mosaic virus (ToMV) | Tobamovirus | Single-stranded RNA rod shaped structure, about 300 nm length and 18 nm radius [27] | S1 | 4 | Capsid protein | Q83482 | 6.4 |
| Tomato mottle mosaic virus (ToMMV) | Tobamovirus | Single-stranded RNA rod-shaped virus particles 300 nm in length [28,29] | S1 | 7 | Capsid protein | T1WEZ3 | 5.7 |
| Tomato spotted wilt virus (TSWV) | Orthotospovirus | Single-stranded RNA roughly spherical shaped with a diameter 80–120 nm and density of 1.207 g/mL [34] | S2 | B2 | Nucleoprotein | O55648 | 58.1 |
| NSs non-structural protein | E1Y5V2 | 19.9 | |||||
| Nucleocapsid protein | A0A0N9H8W3 | 56.7 | |||||
| Putative movement protein | A0A097PIF5 | 30.1 | |||||
| Potato virus Y (PVY) | Potyvirus | Single-stranded RNA, a filamentous, flexuous form, with a length of 730 nm and a diameter of 12 nm [53,54] | S2 | B1 | Putative coat protein | A0A0K2K0B0 | 18.3 |
| B2 | Genome polyprotein | P18247 | 9.3 | ||||
| Southern tomato virus (STV) | Amalgavirus | Double-stranded RNA, shape and size NA [55] | S2 | B2 | Putative coat protein | A0A0K2K0B0 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammadova, R.; Fiume, I.; Bokka, R.; Kralj-Iglič, V.; Božič, D.; Kisovec, M.; Podobnik, M.; Zavec, A.B.; Hočevar, M.; Gellén, G.; et al. Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials 2021, 11, 1922. https://doi.org/10.3390/nano11081922
Mammadova R, Fiume I, Bokka R, Kralj-Iglič V, Božič D, Kisovec M, Podobnik M, Zavec AB, Hočevar M, Gellén G, et al. Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials. 2021; 11(8):1922. https://doi.org/10.3390/nano11081922
Chicago/Turabian StyleMammadova, Ramila, Immacolata Fiume, Ramesh Bokka, Veronika Kralj-Iglič, Darja Božič, Matic Kisovec, Marjetka Podobnik, Apolonija Bedina Zavec, Matej Hočevar, Gabriella Gellén, and et al. 2021. "Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach" Nanomaterials 11, no. 8: 1922. https://doi.org/10.3390/nano11081922
APA StyleMammadova, R., Fiume, I., Bokka, R., Kralj-Iglič, V., Božič, D., Kisovec, M., Podobnik, M., Zavec, A. B., Hočevar, M., Gellén, G., Schlosser, G., & Pocsfalvi, G. (2021). Identification of Tomato Infecting Viruses That Co-Isolate with Nanovesicles Using a Combined Proteomics and Electron-Microscopic Approach. Nanomaterials, 11(8), 1922. https://doi.org/10.3390/nano11081922