Electrode–Electrolyte Interactions in an Aqueous Aluminum–Carbon Rechargeable Battery System
Abstract
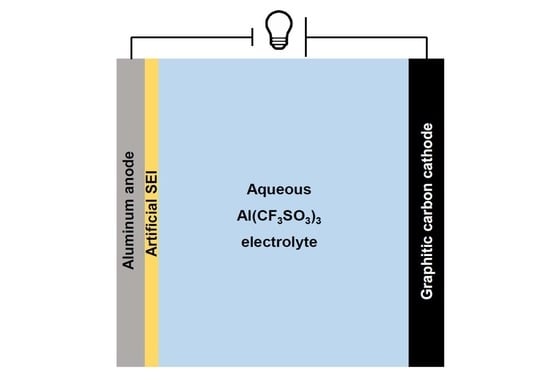
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Materials Synthesis
4.2. Materials Characterization
4.3. Electrochemical Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Smajic, J.; Alazmi, A.; Patole, S.P.; Costa, P.M.F.J. Single-walled carbon nanotubes as stabilizing agents in red phosphorus Li-ion battery anodes. RSC Adv. 2017, 7, 39997–40004. [Google Scholar] [CrossRef] [Green Version]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Wanger, T.C. The lithium future: Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent progress in rechargeable potassium batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Mao, M.; Gao, T.; Hou, S.; Wang, C. A critical review of cathodes for rechargeable Mg batteries. Chem. Soc. Rev. 2018, 47, 8804–8841. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Rombach, G. Raw material supply by aluminium recycling: Efficiency evaluation and long-term availability. Acta Mater. 2013, 61, 1012–1020. [Google Scholar] [CrossRef]
- Bertram, M.; Hryniuk, M.; Kirchner, G.; Pruvost, F. Aluminium Recycling in Europe: The Road to High Quality Products; European Aluminum Association: Brussels, Belgium, 2006. [Google Scholar]
- Bertram, M.; Martchek, K.J.; Rombach, G. Material flow analysis in the aluminum industry. J. Ind. Ecol. 2009, 13, 650–654. [Google Scholar] [CrossRef]
- Bai, Y.; Essehli, R.; Jafta, C.J.; Livingston, K.M.; Belharouak, I. Recovery of cathode materials and aluminum foil using a green solvent. ACS Sustain. Chem. Eng. 2021, 9, 6048–6055. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Zhang, M.; Xiang, L.; Galluzzi, M.; Jiang, C.; Zhang, S.; Li, J.; Tang, Y. Uniform distribution of alloying/dealloying stress for high structural stability of an Al anode in high-areal-density lithium-ion batteries. Adv. Mater. 2019, 31, 1900826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, X.; Zhou, J.; Lu, B. Polyimide/metal-organic framework hybrid for high performance Al-organic battery. Energy Storage Mater. 2020, 31, 58–63. [Google Scholar] [CrossRef]
- Faegh, E.; Ng, B.; Hayman, D.; Mustain, W.E. Practical assessment of the performance of aluminium battery technologies. Nat. Energy 2021, 6, 21–29. [Google Scholar] [CrossRef]
- Smajic, J.; Alazmi, A.; Batra, N.; Palanisamy, T.; Anjum, D.H.; Costa, P.M. Mesoporous reduced graphene oxide as a high capacity cathode for aluminum batteries. Small 2018, 14, 1803584. [Google Scholar] [CrossRef] [Green Version]
- Smajic, J.; Wee, S.; Simoes, F.R.F.; Hedhili, M.N.; Wehbe, N.; Abou-Hamad, E.; Costa, P.M. Capacity retention analysis in aluminum-sulfur batteries. ACS Appl. Energy Mater. 2020, 3, 6805–6814. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, M.; Lin, M.-C.; Yuan, C.; Angell, M.; Huang, L.; Wang, D.-Y.; Zhang, X.; Yang, J.; Hwang, B.-J.; et al. 3D graphitic foams derived from chloroaluminate anion intercalation for ultrafast aluminum-ion battery. Adv. Mater. 2016, 28, 9218–9222. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A novel aluminum-ion battery: Al/AlCl3-[EMIm]Cl/Ni3S2@graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, B.; Ye, D.; Zhu, X.; Lyu, M.; Wang, L. An innovative freeze-dried reduced graphene oxide supported SnS2 cathode active material for aluminum-ion batteries. Adv. Mater. 2017, 29, 1606132. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Luo, H.; Zhou, X.; Liu, Z. Large-sized few-layer graphene enables an ultrafast and long-life aluminum-ion battery. Adv. Energy Mater. 2017, 7, 1700034. [Google Scholar] [CrossRef]
- Leitch, A.C.; Abdelghany, T.M.; Probert, P.M.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Cooke, M.P.; Blake, L.I.; Morse, K.; Rosenmai, A.K. The toxicity of the methylimidazolium ionic liquids, with a focus on M8OI and hepatic effects. Food Chem. Toxicol. 2020, 136, 111069. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Slivac, I.; Srček, V.G. Toxicity mechanisms of ionic liquids. Arch. Ind. Hyg. Toxicol. 2017, 68, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Smajic, J.; Simoes, F.R.F.; Costa, P.M.F.J. How metallic impurities in carbon cathodes affect the electrochemistry of aluminum batteries. ChemElectroChem 2020, 7, 4810–4814. [Google Scholar] [CrossRef]
- Smajic, J.; Alazmi, A.; Costa, P.M.F.J. The role of the binder/solvent pair on the electrochemical performance of aluminium batteries. MRS Adv. 2019, 4, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhang, J.; Guo, J. Avoiding pitfalls in rechargeable aluminum batteries research. ACS Energy Lett. 2019, 4, 2124–2129. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.P.; Azevedo, A.M.; Pinto, P.C.; Saraiva, M.L.M. Environmental impact of ionic liquids: Recent advances in (eco)toxicology and (bio)degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Liu, L.; Yin, J.; Zheng, J.; Zhang, D.; Chen, J.; Archer, L. Proton intercalation/de-intercalation dynamics in vanadium oxides for aqueous aluminum electrochemical cells. Angew. Chem. 2019, 59, 3048–3052. [Google Scholar] [CrossRef]
- Cai, Y.; Kumar, S.; Chua, R.; Verma, V.; Du, Y.; Kou, Z.; Ren, H.; Arora, H.; Srinivasan, M. Bronze-type vanadium dioxide holey nanobelts as high performing cathode material for aqueous aluminium-ion battery. J. Mater. Chem. A 2020, 8, 12716–12722. [Google Scholar] [CrossRef]
- Nandi, S.; Das, S.K. Realizing a low-cost and sustainable rechargeable aqueous aluminum-metal battery with exfoliated graphite cathode. ACS Sustain. Chem. Eng. 2019, 7, 19839–19847. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Wang, C.; Rao, A.M.; Lu, B. Sulfur-assisted large-scale synthesis of graphene microspheres for superior potassium-ion batteries. Energy Environ. Sci. 2021, 14, 965–974. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, V.; Arora, H.; Manalastas, W., Jr.; Srinivasan, M. Rechargeable Al-metal aqueous battery using NaMnHCF as a cathode: Investigating the role of coated-Al anode treatments for superior battery cycling performance. ACS Appl. Energy Mater. 2020, 3, 8627–8635. [Google Scholar] [CrossRef]
- Xu, L.-N.; Zhu, J.-Y.; Lu, M.-X.; Zhang, L.; Chang, W. Electrochemical impedance spectroscopy study on the corrosion of the weld zone of 3Cr steel welded joints in CO2 environments. Int. J. Miner. Metall. Mater. 2015, 22, 500–508. [Google Scholar] [CrossRef]
- Boukamp, B.A. Interpretation of an ‘inductive loop’ in the impedance of an oxygen ion conducting electrolyte/metal electrode system. Solid State Ion. 2001, 143, 47–55. [Google Scholar] [CrossRef]
- Sherwood, P.M. Introduction to studies of aluminum and its compounds by XPS. Surf. Sci. Spectra 1998, 5, 1–3. [Google Scholar] [CrossRef]
- Elmi, C.; Guggenheim, S.; Gieré, R. Surface crystal chemistry of phyllosilicates using X-ray photoelectron spectroscopy: A review. Clays Clay Miner. 2016, 64, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Bagus, P.; Pacchioni, G.; Parmigiani, F. Surface core-level spectroscopy of Cu (100) and Al (100). Phys. Rev. B 1991, 43, 5172. [Google Scholar] [CrossRef]
- Palchan, I.; Crespin, M.; Estrade-Szwarckopf, H.; Rousseau, B. Graphite fluorides: An XPS study of a new type of C-F bonding. Chem. Phys. Lett. 1989, 157, 321–327. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Shen, X.; Wang, H.; Wang, H.; Xia, K.; Yin, Z.; Zhang, Y. Biomass-derived carbon materials: Controllable preparation and versatile applications. Small 2021, 17, 2008079. [Google Scholar] [CrossRef]
- Alazmi, A.; El Tall, O.; Rasul, S.; Hedhili, M.N.; Patole, S.P.; Costa, P.M. A process to enhance the specific surface area and capacitance of hydrothermally reduced graphene oxide. Nanoscale 2016, 8, 17782–17787. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousaive, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J. Definitions of pseudocapacitive materials: A brief review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Alazmi, A.; Rasul, S.; Patole, S.P.; Costa, P.M. Comparative study of synthesis and reduction methods for graphene oxide. Polyhedron 2016, 116, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Alzahrani, A.A. Production of High-Quality Few-Layer Graphene Flakes by Intercalation and Exfoliation; King Abdullah University of Science and Technology: Thuwal, Saudi Arabia, 2017. [Google Scholar]




| Al | O | C | S | F | Cl | |
|---|---|---|---|---|---|---|
| Charged before | 25.2 | 45.8 | 25.0 | 0.4 | 1.6 | 1.9 |
| Charged after | 34.3 | 52.8 | 11.4 | n/a | 0.8 | 0.6 |
| Discharged before | 21.7 | 45.5 | 29.7 | 0.4 | 1.3 | 0.3 |
| Discharged after | 33.7 | 52.4 | 11.4 | n/a | 0.6 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smajic, J.; Alazmi, A.; Wehbe, N.; Costa, P.M.F.J. Electrode–Electrolyte Interactions in an Aqueous Aluminum–Carbon Rechargeable Battery System. Nanomaterials 2021, 11, 3235. https://doi.org/10.3390/nano11123235
Smajic J, Alazmi A, Wehbe N, Costa PMFJ. Electrode–Electrolyte Interactions in an Aqueous Aluminum–Carbon Rechargeable Battery System. Nanomaterials. 2021; 11(12):3235. https://doi.org/10.3390/nano11123235
Chicago/Turabian StyleSmajic, Jasmin, Amira Alazmi, Nimer Wehbe, and Pedro M. F. J. Costa. 2021. "Electrode–Electrolyte Interactions in an Aqueous Aluminum–Carbon Rechargeable Battery System" Nanomaterials 11, no. 12: 3235. https://doi.org/10.3390/nano11123235
APA StyleSmajic, J., Alazmi, A., Wehbe, N., & Costa, P. M. F. J. (2021). Electrode–Electrolyte Interactions in an Aqueous Aluminum–Carbon Rechargeable Battery System. Nanomaterials, 11(12), 3235. https://doi.org/10.3390/nano11123235