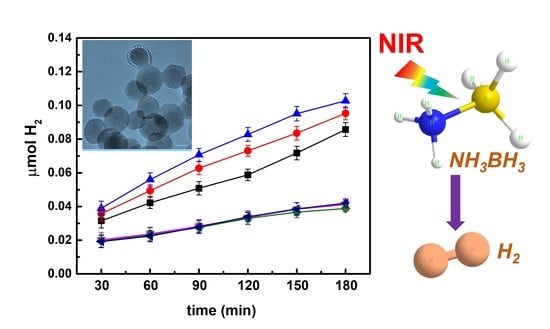
Efficient Near-Infrared-Activated Photocatalytic Hydrogen Evolution from Ammonia Borane with Core-Shell Upconversion-Semiconductor Hybrid Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Photocatalytic H2 Evolution
2.4. Synthesis of NaGdF4:Yb3+/Er3+ UCNPs
2.5. Synthesis of Hydrophobic Core-Shell NaGdF4:Yb3+/Er3+@NaGdF4 UCNPs
2.6. Transformation to Hydrophilic NaGdF4:Yb3+/Er3+@NaGdF4 UCNPs
2.7. Synthesis of NaGdF4:Yb3+/Er3+@NaGdF4@Cu2O UCNPs
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motyka, T.; Zidan, R.; Summers, W.A. Hydrogen Storage: The Key Challenge Facing a Hydrogen Economy. United States Department of Energy 2004. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.194.5361&rep=rep1&type=pdf (accessed on 15 November 2021).
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-Borane and Related Compounds as Dihydrogen Sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-Phase Chemical Hydrogen Storage Materials. Energy Environ. Sci. 2012, 5, 9698. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel Hydrogen Storage Materials: A Review of Lightweight Complex Hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Orimo, S.-I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Orimo, S.-I. Tetrahydroborates as New Hydrogen Storage Materials. Scr. Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Amendola, S.C.; Onnerud, P.; Kelly, M.T.; Petillo, P.J.; Sharp-Goldman, S.L.; Binder, M. A Novel High Power Density Borohydride-Air Cell. J. Power Sources 1999, 84, 130–133. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Sodium Borohydride versus Ammonia Borane, in Hydrogen Storage and Direct Fuel Cell Applications. Energy Environ. Sci. 2009, 2, 627. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Go No-Go Recommendation for Sodium Borohydride for On-Board Vehicular Hydrogen Storage. 2007; NREL/MP-150-42220. Available online: https://www.hydrogen.energy.gov/pdfs/42220.pdf (accessed on 15 November 2021).
- Jo, S.; Verma, P.; Kuwahara, Y.; Mori, K.; Choi, W.; Yamashita, H. Enhanced Hydrogen Production from Ammonia Borane Using Controlled Plasmonic Performance of Au Nanoparticles Deposited on TiO2. J. Mater. Chem. A 2017, 5, 21883–21892. [Google Scholar] [CrossRef]
- Stephens, F.H.; Pons, V.; Tom Baker, R. Ammonia–Borane: The Hydrogen Source Par Excellence? Dalton Trans. 2007, 25, 2613–2626. [Google Scholar] [CrossRef]
- Coskuner, O.; Figen, A.K. Hydro-catalytic Treatment of Organoamine Boranes for Efficient Thermal Dehydrogenation for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 35641–35652. [Google Scholar] [CrossRef]
- Lv, H.; Wei, R.; Guo, X.W.; Sun, L.Z.; Liu, B. Synergistic Catalysis of Binary RuP Nanoclusters on Nitrogen-Functionalized Hollow Mesoporous Carbon in Hydrogen Production from the Hydrolysis of Ammonia Borane, J.P. Chem. Lett. 2021, 12, 696–703. [Google Scholar] [CrossRef]
- Ren, X.Y.; Lv, H.; Yang, S.; Wang, Y.Y.; Li, J.L.; Wei, R.; Xu, D.D.; Liu, B. Promoting Effect of Heterostructured NiO/Ni on Pt Nanocatalysts Toward Catalytic Hydrolysis of Ammonia Borane. J. Phys. Chem. Lett. 2019, 10, 7374–7382. [Google Scholar] [CrossRef]
- Wang, G.Q.; Wang, C.J.; Zhang, H.; Liu, Y.L.; Xu, J. Facile Preparation of Cu-Fe Oxide Nanoplates for Ammonia Borane Decomposition and Tandem Nitroarene Hydrogenation. RSC Adv. 2021, 11, 29920–29924. [Google Scholar] [CrossRef]
- Wei, R.; Chen, Z.; Lv, H.; Zheng, X.; Ge, X.; Sun, L.; Song, K.; Kong, C.; Zhang, W.; Liu, B. Ultrafine RhNi Nanocatalysts Confined in Hollow Mesoporous Carbons for a Highly Efficient Hydrogen Production from Ammonia Borane. Inorg. Chem. 2021, 60, 6820–6828. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Grape, E.S.; Gaggioli, C.A.; Tayal, A.; Dharanipragada, A.; Willhammar, T.; Inge, A.K.; Zou, X.D.; Liu, B.; et al. A Tunable Multivariate Metal-Organic Framework as a Platform for Designing Photocatalysts. J. Am Chem. Soc. 2021, 143, 6333–6338. [Google Scholar] [CrossRef]
- He, J.H.; Yao, Z.D.; Xiao, X.Z.; Chen, W.Z.; Huang, Z.W.; Fan, X.L.; Dong, Z.; Huang, X.; Wang, X.C.; Chen, M.; et al. Heterostructured Ni/NiO Nanoparticles on 1D Porous MoOx for Hydrolysis of Ammonia Borane. ACS Appl. Energy Mater. 2021, 4, 1208–1217. [Google Scholar] [CrossRef]
- Zhang, L.; Oishi, T.; Gao, L.Z.; Hu, S.Y.; Yang, L.L.; Li, W.; Wu, S.J.; Shang, R.; Yamamoto, Y.; Li, S.H.; et al. Catalytic Dehydrogenation of Ammonia Borane Mediated by a Pt(0)/Borane Frustrated Lewis Pair: Theoretical Design. ChemPhysChem 2020, 21, 2573–2578. [Google Scholar] [CrossRef]
- Nagyhazi, M.; Turczel, G.; Anastas, P.T.; Tuba, R. Highly Efficient Ammonia Borane Hydrolytic Dehydrogenation in Neat Water Using Phase-Labeled CAAC-Ru Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16097–16103. [Google Scholar] [CrossRef]
- Pei, P.; Cannon, M.; Quan, G.; Kjeang, E. Effective Hydrogen Release from Ammonia Borane and Sodium Borohydride Mixture Through Homopolar Based Dehydrocoupling Driven by Intermolecular Interaction and Restrained Water Supply. J. Mater. Chem. A 2020, 8, 19050–19056. [Google Scholar] [CrossRef]
- Yao, Q.L.; Ding, Y.Y.; Lu, Z.H. Noble-metal-free Nanocatalysts for Hydrogen Generation from Boron- and Nitrogen-based Hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.Y.; Zhang, X.; Wang, Z.M.; Yu, R.B. A MOF-derived CuCo(O)@Carbon-nitrogen Framework as an Efficient Synergistic Catalyst for the Hydrolysis of Ammonia Borane. Inorg. Chem. Front. 2020, 7, 2043–2049. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.L.; Zhang, D.; Li, G.D.; Meng, W.; Wang, D.; Cao, Z.Q.; Zhang, K.; Wu, S.W. Co-Mo-B Nanoparticles Supported on Carbon Cloth as Effective Catalysts for the Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2020, 45, 14418–14427. [Google Scholar] [CrossRef]
- Principe, G.; Agresti, F.; Maddalena, A.; Russo, S.L. The Problem of Solid-State Hydrogen Storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.-F.; Leardini, F.; Ferrer, I.J.; Fernandez, J.-F.; Guo, Z.-X.; Sánchez, C. Hydrogen Absorption/Desorption Mechanism in Potassium Alanate (KAlH4) and Enhancement by TiCl3 Doping. J. Phys. Chem. C 2009, 113, 6845–6851. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.-I.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Felderhoff, M.; Streukens, G. Hydrogen Storage in Complex Metal Hydrides. J. Serb. Chem. Soc. 2009, 74, 183–196. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Yao, J.; Guo, Z.X. Reaction Paths between LiNH2and LiH with Effects of Nitrides. J. Phys. Chem. B 2007, 111, 12531–12536. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yong, C.K.; Wu, G.; Chen, P.; Shaw, W.; Karkamkar, A.; Autrey, T.; Jones, M.O.; Johnson, S.R.; Edwards, P.P.; et al. High-Capacity Hydrogen Storage in Lithium and Sodium Amidoboranes. Nat. Mater. 2008, 7, 138–141. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S.-I. Destabilization of Li-Based Complex Hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase Boundaries and Reversibility of LiBH4/MgH2 Hydrogen Storage Material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Vajo, J.J.; Olson, G.L. Hydrogen Storage in Destabilized Chemical Systems. Scripta Mater. 2007, 56, 829–834. [Google Scholar] [CrossRef]
- Yu, X.B.; Guo, Y.H.; Sun, D.L.; Yang, Z.X.; Ranjbar, A.; Guo, Z.P.; Liu, H.K.; Dou, S.X. A Combined Hydrogen Storage System of Mg(BH4)2−LiNH2with Favorable Dehydrogenation. J. Phys. Chem. C 2010, 114, 4733–4737. [Google Scholar] [CrossRef]
- Bosenberg, U.; Kim, J.; Gosslar, D.; Eigen, N.; Jensen, T.; Colbe, J.B.V.; Zhou, Y.; Dahms, M.; Kim, D.; Günther, R. Role of Additives in LiBH4–MgH2 Reactive Hydride Composites for Sorption Kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef] [Green Version]
- Christian, M.L.; Aguey-Zinsou, K.F. Core–Shell Strategy Leading to High Reversible Hydrogen Storage Capacity for NaBH4. ACS Nano 2012, 6, 7739–7751. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhu, M.; Yang, X.; Zhang, Y.; Whangbo, M.-H.; Li, B.; Huang, B. Continual Injection of Photoinduced Electrons Stabilizing Surface Plasmon Resonance of Non-Elemental-Metal Plasmonic Photocatalyst CdS/WO3−x for Efficient Hydrogen Generation. Appl. Catal. B Environ. 2018, 226, 10–15. [Google Scholar] [CrossRef]
- Ye, C.; Wang, R.; Wang, H.; Jiang, F. The High Photocatalytic Efficiency and Stability of LaNiO3/g-C3N4 Heterojunction Nanocomposites for Photocatalytic Water Splitting to Hydrogen. BMC Chem. 2020, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Fujishima, M.; Kobayashi, H. Photodeposition of Metal Sulfide Quantum Dots on Titanium (IV) Dioxide and the Applications to Solar Energy Conversion. Chem. Soc. Rev. 2011, 40, 4232–4243. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, T.; Xu, Q.; Li, D.; Meng, S.; Chen, M. Perovskite Oxide Ultrathin with Significantly Enhanced Photocatalytic Activity towards the Photodegradation of Tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar] [CrossRef]
- Paramanik, L.; Reddy, K.H.; Sultana, S.; Parida, K. Architecture of biperovskite-based LaCrO3/PbTiO3 p–n heterojunction with a strong interface for enhanced charge anti-recombination process and visible light-induced photocatalytic reactions. Inorg. Chem. 2018, 57, 15133–15148. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, J.; Jia, L.; Fang, W. Investigations on the Effect of Cu2+/Cu+ Redox Couples and Oxygen Vacancies on Photocatalytic Activity of Treated LaNi1−xCuxO3 (x = 0.1, 0.4, 0.5). Int. J. Hydrogen Energy 2010, 35, 12733–12740. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Q.; Song, Q.; Pan, X.; Jia, L. Novel Microbial Synthesis of Cu Doped LaCoO3 Photocatalyst and its Highly Efficient Hydrogen Production from Formaldehyde Solution under Visible Light Irradiation. Fuel 2015, 140, 267–274. [Google Scholar] [CrossRef]
- Wang, J.; Cui, C.; Kong, Q.; Ren, C.; Li, Z.; Qu, L.; Zhang, Y.; Jiang, K. Mn-Doped g-C3N4 Nanoribbon for Efficient Visible-light Photocatalytic Water Splitting Coupling with Methylene Blue Degradation. ACS Sustain. Chem. Eng. 2018, 6, 8754–8761. [Google Scholar] [CrossRef]
- Gupta, A.; Chemelewski, W.D.; Buddie Mullins, C.; Goodenough, J.B. High-rate Oxygen Evolution Reaction on Al-Doped LiNiO2. Adv. Mater. 2015, 27, 6063–6067. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Fang, T.; Li, Z.; Zou, Z. Promoted Photoelectrochemical Activity of BiVO4 coupled with LaFeO3 and LaCoO3. Res. Chem. Intermediat. 2018, 44, 1013–1024. [Google Scholar] [CrossRef]
- Dhinesh Kumar, R.; Thangappan, R.; Jayavel, R. Synthesis and Characterization of LaFeO3/TiO2 Nanocomposites for Visible Light Photocatalytic Activity. J. Phys. Chem. Solids 2017, 101, 25–33. [Google Scholar] [CrossRef]
- Ling, F.; Anthony, O.C.; Xiong, Q.; Luo, M.; Pan, X.; Jia, L.; Huang, J.; Sun, D.; Li, Q. PdO/LaCoO3 Heterojunction Photocatalysts for Highly Hydrogen Production from Formaldehyde Aqueous Solution under Visible Light. Int. J. Hydrogen Energy 2016, 41, 6115–6122. [Google Scholar] [CrossRef]
- Chu, J.; Han, X.; Yu, Z.; Du, Y.; Song, B.; Xu, P. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production on CdS/Cu7S4/g-C3N4 Ternary Heterostructures. ACS Appl. Mater. Inter. 2018, 10, 20404–20411. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Liu, G.; Ye, J. A Highly Durable p-LaFeO3/n-Fe2O3 Photocell for Effective Water Splitting under Visible Light. Chem. Commun. 2015, 51, 3630–3633. [Google Scholar] [CrossRef]
- Jia, L.; Li, J.; Fang, W. Enhanced Visible-Light Active C and Fe Co-Doped LaCoO3 for Reduction of Carbon Dioxide. Catal. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Li, W.; Guo, Q.; Gao, H.; You, Y.; Li, Y.; Cui, Z.; Jiang, K.-C.; Long, H.; et al. Nitrogen-Doped Perovskite as a Bifunctional Cathode Catalyst for Rechargeable Lithium-Oxygen Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5543–5550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiang, W.; Hua, S.; Zhao, H.; Zhang, L.; Sun, Z. Constructing Bulk Defective Perovskite SrTiO3 Nanocubes for High Performance Photocatalysts. Nanoscale 2016, 8, 16963–16968. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Porous Nanoplate-like Tungsten Trioxide/Reduced Graphene Oxide Catalyst for Sonocatalytic Degradation and Photocatalytic Hydrogen Production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Aksoy, M.; Metin, Ö. Pt Nanoparticles Supported on Mesoporous Graphitic Carbon Nitride as Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Nano Mater. 2020, 3, 6836–6846. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Huang, Y.; Min, X.; Kong, C.; Tang, Y.; Liu, B. Atomically ordered Rh2P catalysts anchored within hollow mesoporous carbon for efficient hydrogen production. Chem. Commun. 2021, 57, 12345–12348. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhao, X.; Luo, W.; Zhang, Y.; Wang, Y.; Fan, G. Bagasse-derived Carbon-supported Ru nanoparticles as Catalyst for Efficient Dehydrogenation of Ammonia Borane. ChemNanoMat 2020, 6, 1251–1259. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Jiang, R.; Zheng, X.; Liu, P. Ru Nanoparticles Immobilized on Chitosan as Effective Catalysts for Boosting NH3BH3 Hydrolysis. ChemCatChem 2021, 13, 4142–4150. [Google Scholar] [CrossRef]
- Paul, A.; Musgrave, C.B. Catalyzed Dehydrogenation of Ammonia–Borane by Iridium Dihydrogen Pincer Complex Differs from Ethane Dehydrogenation. Angew. Chem. 2007, 119, 8301–8304. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Du, X.; Sun, S.; Yu, X.; Zhang, X.; Lu, Z.; Li, L.; Yang, X. Hydrogen production from ammonia borane hydrolysis catalyzed by non-noble metal-based materials: A review. J. Mater. Sci. 2021, 56, 2856–2878. [Google Scholar] [CrossRef]
- Zhan, W.-W.; Zhu, Q.-L.; Xu, Q. Dehydrogenation of Ammonia Borane by Metal Nanoparticle Catalysts. ACS Catal. 2016, 6, 6892–6905. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, L.; Dushimimana, F.; Zhao, J.; Wang, S.; Han, J.; Zhu, X.; Liu, X.; Ge, Q.; et al. Visible-Light-Driven Multichannel Regulation of Local Electron Density to Accelerate Activation of O–H and B–H Bonds for Ammonia Borane Hydrolysis. ACS Catal. 2020, 10, 14903–14915. [Google Scholar] [CrossRef]
- Xi, P.; Chen, F.; Xie, G.; Ma, C.; Liu, H.; Shao, C.; Wang, J.; Xu, Z.; Xu, X.; Zeng, Z. Surfactant Free RGO/Pd Nanocomposites as Highly Active Heterogeneous Catalysts for the Hydrolytic Dehydrogenation of Ammonia Borane for Chemical Hydrogen Storage. Nanoscale 2012, 4, 5597–5601. [Google Scholar] [CrossRef]
- Alyami, N.M.; LaGrow, A.P.; Anjum, D.H.; Guan, C.; Miao, X.; Sinatra, L.; Yuan, D.-J.; Mohammed, O.F.; Huang, K.-W.; Bakr, O.M. Synthesis and Characterization of Branched fcc/hcp Ruthenium Nanostructures and their Catalytic Activity in Ammonia Borane Hydrolysis. Cryst. Growth Des. 2018, 18, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.; Zhu, Y.; Mi, G.; Du, X.; Dong, Y. Shape Selective Fabrication of Cu Nanostructures: Contrastive Study of Catalytic Ability for Hydrolytically Releasing H2 from Ammonia Borane. Renew. Energy 2018, 118, 146–151. [Google Scholar] [CrossRef]
- Yao, K.; Zhao, C.; Wang, N.; Li, T.; Lu, W.; Wang, J. An Aqueous Synthesis of Porous PtPd Nanoparticles with Reversed Bimetallic Structures for Highly Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. Nanoscale 2020, 12, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, F.; Yang, S.; Martinez Moro, M.; Ramirez, M.d.l.A.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic Synergy in CoPt Nanocatalysts Stabilized by “Click” Dendrimers for Evolution of Hydrogen from Hydrolysis of Ammonia Borane. ACS Catal. 2019, 9, 1110–1119. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, R.; Li, Q.; Zeng, F.; Zheng, X.; Xu, Z.; Chen, C.; Peng, J. The Hydrolysis of Ammonia Borane Catalyzed by NiCoP/OPC-300 Nanocatalysts: High Selectivity and Efficiency, and Mechanism. Green Chem. 2019, 21, 850–860. [Google Scholar] [CrossRef]
- Wang, C.; Tuninetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of Ammonia Borane over Ni/ZIF-8 Nanocatalyst: High Efficiency, Mechanism, and Controlled Hydrogen Release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Lu, W.; Zhang, J.; Zhang, L. Efficient Hydrolysis of Ammonia Borane for Hydrogen Evolution Catalyzed by Plasmonic Ag@Pd Core–Shell Nanocubes. ACS Sustain. Chem. Eng. 2020, 8, 12366–12377. [Google Scholar] [CrossRef]
- Yan, J.-M.; Zhang, X.-B.; Han, S.; Shioyama, H.; Xu, Q. Iron-Nanoparticle-Catalyzed Hydrolytic Dehydrogenation of Ammonia Borane for Chemical Hydrogen Storage. Angew. Chem. Int. Edit. 2008, 47, 2287–2289. [Google Scholar] [CrossRef]
- Metin, Ö.; Mazumder, V.; Özkar, S.; Sun, S. Monodisperse Nickel Nanoparticles and Their Catalysis in Hydrolytic Dehydrogenation of Ammonia Borane. J. Am. Chem. Soc. 2010, 132, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Z.; Li, M.; Zhou, X.; Lu, H. Amine-Capped Co Nanoparticles for Highly Efficient Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2014, 6, 13191–13200. [Google Scholar] [CrossRef]
- Li, D.; Wehrung, J.-F.; Zhao, Y. Gold Nanoparticle-Catalyzed Photosensitized Water Reduction for Hydrogen Generation. J. Mater. Chem. A 2015, 3, 5176–5182. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.; Badreldin, A.; Chava, R.; Do, J.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.W.; Lu, Z.H.; Hu, Y.J.; Zhang, Z.J.; Shi, W.M.; Chen, X.S.; Wang, T.T. Facile in situ Synthesis of Copper Nanoparticles Supported on Reduced Graphene Oxide for Hydrolytic Dehydrogenation of Ammonia Borane. RSC Adv. 2014, 4, 13749–13752. [Google Scholar] [CrossRef]
- Li, J.L.; Ren, X.Y.; Lv, H.; Wang, Y.Y.; Li, Y.F.; Liu, B. Highly Efficient Hydrogen Production from Hydrolysis of Ammonia Borane Over Nanostructured Cu@CuCoOx Supported on Graphene Oxide. J. Hazard. Mater. 2020, 391, 122199. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yano, K.; Xu, Q.; Fukuzumi, S. Cu/Co3O4 Nanoparticles as Catalysts for Hydrogen Evolution from Ammonia Borane by Hydrolysis. J. Phys. Chem. C 2010, 114, 16456–16462. [Google Scholar] [CrossRef]
- Yao, Q.L.; Huang, M.; Lu, Z.H.; Yang, Y.W.; Zhang, Y.X.; Chen, X.S.; Yang, Z. Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper Nanostructures for Hydrogen Generation. Dalton Trans. 2015, 44, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.H.; Li, J.P.; Zhu, A.L.; Yao, Q.L.; Huang, W.; Zhou, R.Y.; Zhou, R.F.; Chen, X.S. Catalytic Hydrolysis of Ammonia Borane via Magnetically Recyclable Copper Iron Nanoparticles for Chemical Hydrogen Storage. Int. J. Hydrogen Energy 2013, 38, 5330–5337. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.W.; Hua, W.; Bettinelli, M.; Liu, X.G. Lanthanide-Activated Phosphors Based on 4f–5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, B.Y.; Jin, D.Y.; Liu, X.G. Controlling Upconversion Nanocrystals for Emerging Applications. Nat. Nanotechnol. 2015, 10, 924–936. [Google Scholar] [CrossRef]
- Qin, X.; Xu, J.H.; Wu, Y.M.; Liu, X.G. Energy-Transfer Editing in Lanthanide-Activated Upconversion Nanocrystals: A Toolbox for Emerging Applications. ACS Cent. Sci. 2019, 5, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F.Y. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Qju, H.L.; Prasad, P.N.; Chen, X.Y. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Du, S.R.; Zheng, X.Y.; Lyu, G.M.; Sun, L.D.; Li, L.D.; Zhang, P.Z.; Zhang, C.; Yan, C.H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Snoke, D.; Wolfe, J.P.; Mysyrowicz, A. Quantum Saturation of a Bose-Gas; Excitons in Cu2O. Phys. Rev. Lett. 1987, 59, 827–830. [Google Scholar] [CrossRef]
- Snoke, D. Coherent Exciton Waves. Science 1996, 273, 1351–1352. [Google Scholar] [CrossRef]
- Jolk, A.; Klingshirn, C.F. Linear and Nonlinear Excitonic Absorption and Photoluminescence Spectra in Cu2O: Line Shape Analysis and Exciton Drift. Phys. Status Solidi B 1998, 206, 841–850. [Google Scholar] [CrossRef]
- Zou, Y.T.; Hu, Y.Z.; Uhrich, A.; Shen, Z.W.; Peng, B.X.; Ji, Z.Y.; Muhler, M.; Zhao, G.X.; Wang, X.K.; Xu, X.X. Steering Accessible Oxygen Vacancies for Alcohol Oxidation Over Defective Nb2O5 Under Visible Light Illumination. Appl. Catal. B. 2021, 298, 120584. [Google Scholar] [CrossRef]
- Li, B.F.; Hong, J.H.; Ai, Y.J.; Hu, Y.Z.; Shen, Z.W.; Li, S.J.; Zou, Y.T.; Zhang, S.; Wang, X.K.; Zhao, G.X.; et al. Visible-Near-Infrared-Light-Driven Selective Oxidation of Alcohols Over Nanostructured Cu Doped SrTiO3 in Water Under Mild Condition. J. Catal. 2021, 399, 142–149. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Xi, Z.S.; Wu, Z.Y.; Lu, G.L.; Huang, X.B. Visible-Light-Induced Selective Oxidation of Amines into Imines Over UiO-66-NH2@Au@COF Core-Shell Photocatalysts. ACS Sustain. Chem. Eng. 2021, 9, 12623–12633. [Google Scholar] [CrossRef]
- Lu, G.L.; Huang, X.B.; Li, Y.; Zhao, G.X.; Pang, G.S.; Wang, G. Covalently Integrated Core-Shell MOF@COF Hybrids as Efficient Visible-Light-Driven Photocatalysts for Selective Oxidation of Alcohols. J. Energy Chem. 2020, 43, 8–15. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelista, A.J.; Ivanchenko, M.; Jing, H. Efficient Near-Infrared-Activated Photocatalytic Hydrogen Evolution from Ammonia Borane with Core-Shell Upconversion-Semiconductor Hybrid Nanostructures. Nanomaterials 2021, 11, 3237. https://doi.org/10.3390/nano11123237
Evangelista AJ, Ivanchenko M, Jing H. Efficient Near-Infrared-Activated Photocatalytic Hydrogen Evolution from Ammonia Borane with Core-Shell Upconversion-Semiconductor Hybrid Nanostructures. Nanomaterials. 2021; 11(12):3237. https://doi.org/10.3390/nano11123237
Chicago/Turabian StyleEvangelista, Andrew J., Mariia Ivanchenko, and Hao Jing. 2021. "Efficient Near-Infrared-Activated Photocatalytic Hydrogen Evolution from Ammonia Borane with Core-Shell Upconversion-Semiconductor Hybrid Nanostructures" Nanomaterials 11, no. 12: 3237. https://doi.org/10.3390/nano11123237
APA StyleEvangelista, A. J., Ivanchenko, M., & Jing, H. (2021). Efficient Near-Infrared-Activated Photocatalytic Hydrogen Evolution from Ammonia Borane with Core-Shell Upconversion-Semiconductor Hybrid Nanostructures. Nanomaterials, 11(12), 3237. https://doi.org/10.3390/nano11123237