Mechanochemically Synthetized PAN-Based Co-N-Doped Carbon Materials as Electrocatalyst for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Reagents
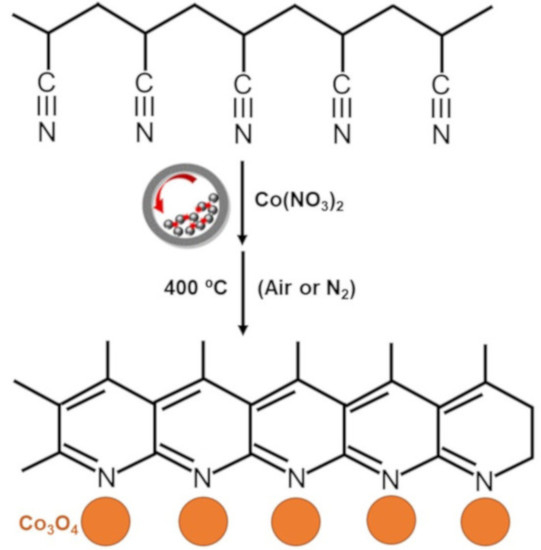
2.2. Synthesis of Co-N-Doped Carbon Catalyst
2.3. Material Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
OER Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badreldin, A.; Abusrafa, A.E.; Abdel-Wahab, A. Oxygen-deficient perovskites for oxygen evolution reaction in alkaline media: A review. Emergent Mater. 2020, 3, 567–590. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-based metal-free ORR electrocatalysts for fuel cells: Past, present, and future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Delgado, O.; Puente-Santiago, A.R.; Cano, M.; Giner-Casares, J.J.; Metta-Magaña, A.J.; Echegoyen, L. Facile synthesis of C 60-nano materials and their application in high-performance water splitting electrocatalysis. Sustain. Energy Fuels 2020, 4, 2900–2906. [Google Scholar] [CrossRef]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Huang, Z.F.; Wang, J.; Peng, Y.; Jung, C.Y.; Fisher, A.; Wang, X. Design of efficient bifunctional oxygen reduction/evolution electrocatalyst: Recent advances and perspectives. Adv. Energy Mater. 2017, 7, 1700544. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, M.A.; Puente Santiago, A.R.; Hong, Y.; Zhang, N.; Cano, M.; Rodríguez-Castellón, E.; Echegoyen, L.; Sreenivasan, S.T.; Noveron, J.C. Tuning of Trifunctional NiCu Bimetallic Nanoparticles Confined in a Porous Carbon Network with Surface Composition and Local Structural Distortions for the Electrocatalytic Oxygen Reduction, Oxygen and Hydrogen Evolution Reactions. J. Am. Chem. Soc. 2020, 142, 14688–14701. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: An application-inspired renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Song, M.; Song, Y.; Sha, W.; Xu, B.; Guo, J.; Wu, Y. Recent Advances in Non-Precious Transition Metal/Nitrogen-doped Carbon for Oxygen Reduction Electrocatalysts in PEMFCs. Catalysts 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.J. Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: Recent advances. Curr. Opin. Green Sustain. Chem. 2020, 27, 100398. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-Noble-Metal-Based Electrocatalysts toward the Oxygen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Sun, X. Recent progress on earth abundant electrocatalysts for oxygen evolution reaction (OER) in alkaline medium to achieve efficient water splitting—A review. J. Power Sources 2018, 400, 31–68. [Google Scholar] [CrossRef]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Davis, D.J.; Limmer, S.J.; Burton, P.D.; Brumbach, M.T. Electrodeposited Ni x Co 3−x O 4 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun. 2015, 51, 9511–9514. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, R.; Candelaria, S.L.; Wang, J.; Liu, Q.; Uchaker, E.; Cao, G. Phosphorus/sulfur Co-doped porous carbon with enhanced specific capacitance for supercapacitor and improved catalytic activity for oxygen reduction reaction. J. Power Sources 2016, 314, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Vigil, J.A.; Lambert, T.N. Nanostructured cobalt phosphide-based films as bifunctional electrocatalysts for overall water splitting. RSC Adv. 2015, 5, 105814–105819. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Cano, M.; Garcia-Garcia, F.J.; Rodríguez-Padrón, D.; González-Elipe, A.R.; Giner-Casares, J.J.; Luque, R. Ultrastable CoxSiyOz Nanowires by Glancing Angle Deposition with Magnetron Sputtering as Novel Electrocatalyst for Water Oxidation. ChemCatChem 2019, 11, 6111–6115. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, X.; Lin, H.; Zhang, P.; Gao, Q.; Li, W. Carbon-based nanomaterials as sustainable noble-metal-free electrocatalysts. Front. Chem. 2019, 7, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.; Zhu, L.; Chen, H.; Qu, J.; Dai, L. Recent Advances in Carbon-Based Metal-Free Electrocatalysts. Adv. Mater. 2019, 31, 1806403. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Padrón, D.; Algarra, M.; Tarelho, L.A.; Frade, J.; Franco, A.; de Miguel, G.; Luque, R. Catalyzed microwave-assisted preparation of carbon quantum dots from lignocellulosic residues. ACS Sustain. Chem. Eng. 2018, 6, 7200–7205. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Luque, R.; Muñoz-Batista, M.J. Waste-derived Materials: Opportunities in Photocatalysis. Top. Curr. Chem. 2020, 378, 3. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Cano, M.; Calsonaro, F.; Puente Santiago, A.R.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Berlier, G.; Cravotto, G.; Martina, K.; Luque, R. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolutuin reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels 2021. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Imam, M.A.; Santiago, A.R.P.; Rodriguez, A.; Alvarado-Tenorio, B.; Bernal, R.; Noveron, J.C. Spent tea leaves templated synthesis of highly active and durable cobalt-based trifunctional versatile electrocatalysts for hydrogen and oxygen evolution and oxygen reduction reactions. Green Chem. 2020, 22, 6967–6980. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Yi, J.D.; Xu, R.; Wu, Q.; Zhang, T.; Zang, K.T.; Luo, J.; Cao, R. Atomically dispersed iron–nitrogen active sites within porphyrinic triazine-based frameworks for oxygen reduction reaction in both alkaline and acidic media. ACS Energy Lett. 2018, 3, 883–889. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-Nitrogen-Doped Carbon Materials as Highly Efficient Catalysts: Progress and Rational Design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Ding, J.; Ji, S.; Wang, H.; Pollet, B.G.; Wang, R. Mesoporous CoS/N-doped Carbon as HER and ORR Bifunctional Electrocatalyst for Water Electrolyzers and Zinc-Air Batteries. ChemCatChem 2019, 11, 1026–1032. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the Role of Quaternary-N of BINOL Covalent Triazine-Based Frameworks in Oxygen Reduction and Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2020, 12, 44689–44699. [Google Scholar] [CrossRef]
- Leal-Rodríguez, C.; Rodríguez-Padrón, D.; Alothman, Z.A.; Cano, M.; Giner-Casares, J.J.; Muñoz-Batista, M.J.; Luque, R. Thermal and light irradiation effects on the electrocatalytic performance of hemoglobin modified Co 3 O 4-gC 3 N 4 nanomaterials for the oxygen evolution reaction. Nanoscale 2020, 12, 8477–8484. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Yang, J.; Ji, M.; Yu, J.; Wang, M.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Preparation, Stabilization and Carbonization of Novel Polyacrylonitrile-Based Carbon Fiber Precursors. Polymers 2019, 11, 1150. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Nandi, M.; Maruyama, J.; Oka, T.; Tsujimoto, T.; Kondoh, K.; Uyama, H. Fabrication of mesoporous polymer monolith: A template-free approach. Chem. Commun. 2011, 47, 7422–7424. [Google Scholar] [CrossRef]
- Maghe, M.; Creighton, C.; Henderson, L.C.; Huson, M.G.; Nunna, S.; Atkiss, S.; Fox, B.L. Using ionic liquids to reduce energy consumption for carbon fibre production. J. Mater. Chem. A 2016, 4, 16619–16626. [Google Scholar] [CrossRef]
- Cho, S.P.; Jang, S.; Jo, H.N.; Lee, S.A.; Bae, S.; Lee, S.H.; Kim, T.W. One step synthesis of Au nanoparticle-cyclized polyacrylonitrile composite films and their use in organic nano-floating gate memory applications. J. Mater. Chem. C 2016, 4, 1511–1516. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Lian, F.; Liang, J. Effect of the oxygen-induced modification of polyacrylonitrile fibers during thermal-oxidative stabilization on the radial microcrystalline structure of the resulting carbon fibers. Polym. Degrad. Stab. 2013, 98, 2259–2267. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Vassiljeva, V.; Merisalu, M.; Kook, M.; Treshchalov, A.; Tammeveski, K. Electrocatalysts for oxygen reduction reaction based on electrospun polyacrylonitrile, styrene–acrylonitrile copolymer and carbon nanotube composite fibres. J. Mater. Sci. 2019, 54, 11618–11634. [Google Scholar] [CrossRef]
- Shu, Y.; Maruyama, J.; Iwasaki, S.; Maruyama, S.; Shen, Y.; Uyama, H. Fabrication of N-doped and shape-controlled porous monolithic carbons from polyacrylonitrile for supercapacitors. RSC Adv. 2017, 7, 43172–43180. [Google Scholar] [CrossRef] [Green Version]
- Alba-Molina, D.; Puente-Santiago, A.R.; Giner-Casares, J.J.; Martín-Romero, M.T.; Camacho, L.; Luque, R.; Cano, M. Citrate-stabilized gold nanoparticles as high-performance electrocatalysts: The role of size in the electroreduction of oxygen. J. Phys. Chem. C 2019, 123, 9807–9812. [Google Scholar] [CrossRef]
- Alba-Molina, D.; Puente-Santiago, A.R.; Giner-Casares, J.J.; Rodríguez-Castellón, E.; Martín-Romero, M.T.; Camacho, L.; Cano, M. Tailoring the ORR and HER electrocatalytic performances of gold nanoparticles through metal–ligand interfaces. J. Mater. Chem. A 2019, 7, 20425–20434. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, R.; Wang, H.; Linkov, V.; Ji, S. Evolution of nanoscale amorphous, crystalline and phase-segregated PtNiP nanoparticles and their electrocatalytic effect on methanol oxidation reaction. Phys. Chem. Chem. Phys. 2014, 16, 3593–3602. [Google Scholar] [CrossRef]
- Sharifi, T.; Hu, G.; Jia, X.; Wagberg, T. Formation of active sites for oxygen reduction reactions by transformation of nitrogen functionalities in nitrogen-doped carbon nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Hauser, J.L. Ni Foam-Supported Fe-Doped β-Ni (OH)2 Nanosheets Show Ultralow Overpotential for Oxygen Evolution Reaction. ACS Energy Lett. 2019, 4, 622–628. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, Z.; Zhang, H.; Wu, P.; Cai, C. Active site structures in nitrogen-doped carbon-supported cobalt catalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8, 32875–32886. [Google Scholar] [CrossRef]
- Bähr, A.; Moon, G.H.; Tüysüz, H. Nitrogen-Doped Mesostructured Carbon-Supported Metallic Cobalt Nanoparticles for Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2019, 2, 6672–6680. [Google Scholar] [CrossRef] [Green Version]
- Tammeveski, K.; Arulepp, M.; Tenno, T.; Ferrater, C.; Claret, J. Oxygen electroreduction on titanium-supported thin Pt films in alkaline solution. Electrochim. Acta 1997, 42, 2961–2967. [Google Scholar] [CrossRef]
- Park, S.M.; Ho, S.; Aruliah, S.; Weber, M.F.; Ward, C.A.; Venter, R.D.; Srinivasan, S. Electrochemical reduction of oxygen at platinum electrodes in KOH solutions-temperature and concentration effects. J. Electrochem. Soc. 1986, 133, 1641. [Google Scholar] [CrossRef]
- Couturier, G.; Kirk, D.W.; Hyde, P.J.; Srinivasan, S. Electrocatalysis of the hydrogen oxidation and of the oxygen reduction reactions of Pt and some alloys in alkaline medium. Electrochim. Acta 1987, 32, 995–1005. [Google Scholar] [CrossRef]
- Zinola, C.F.; Luna, A.C.; Triaca, W.E.; Arvia, A.J. Kinetics and mechanism of the electrochemical reduction of molecular oxygen on platinum in KOH: Influence of preferred crystallographic orientation. J. Appl. Electrochem. 1994, 24, 531–541. [Google Scholar] [CrossRef]
- Lemoine, K.; Lhoste, J.; Hémon-Ribaud, A.; Heidary, N.; Maisonneuve, V.; Guiet, A.; Kornienko, N. Investigation of Amorphous Mixed-Metal (Oxy) Fluorides as a New Class of Water Oxidation Electrocatalysts. Chem. Sci. 2019, 10, 9209–9218. [Google Scholar] [CrossRef]
- Negahdar, L.; Zeng, F.; Palkovits, S.; Broicher, C.; Palkovits, R. Mechanistic Aspects of the Electrocatalytic Oxygen Evolution Reaction over Ni− Co Oxides. ChemElectroChem 2019, 6, 5588–5595. [Google Scholar] [CrossRef]
- Zhao, J.; Cano, M.; Giner-Casares, J.J.; Luque, R.; Xu, G. Electroanalytical methods and their hyphenated techniques for novel ion battery anode research. Energy Environ. Sci. 2020, 13, 2618–2656. [Google Scholar] [CrossRef]
- Feng, X.; Bo, X.; Guo, L. CoM (M = Fe, Cu, Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 2018, 389, 249–259. [Google Scholar] [CrossRef]
- Yang, L.; Feng, S.; Xu, G.; Wei, B.; Zhang, L. Electrospun MOF-Based FeCo Nanoparticles Embedded in Nitrogen-Doped Mesoporous Carbon Nanofibers as an Efficient Bifunctional Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Zinc-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 5462–5475. [Google Scholar] [CrossRef]





| Sample | C1s (%At. Conc.) | O1s (%At. Conc.) | N1s (%At. Conc.) | Co2p (%At. Conc.) | Co/C Ratio |
|---|---|---|---|---|---|
| PAN-A | 35.5 | 28.2 | 36.3 | ND. | |
| PAN-N | 30.3 | 25.9 | 43.8 | ND. | |
| 3%Co-PAN-A | 16.5 | 52.2 | 7.9 | 23.4 | 1.4 |
| 3%Co-PAN-N | 11.8 | 53.2 | 10.2 | 24.8 | 2.1 |
| 10%Co-PAN-A | 14.2 | 58.2 | 1.8 | 25.8 | 1.8 |
| 10%Co-PAN-N | 13.8 | 59.3 | 0.7 | 26.2 | 1.9 |
| Catalyst | SBET [a] (m2/g) | VBJH [b] (cm3/g) | DBJH [c] (nm) |
|---|---|---|---|
| PAN milled | 12.53 | 0.012 | 65 |
| PAN-N | 16.48 | 0.014 | 63 |
| PAN-A/3%Co | 8.79 | 0.025 | 11 |
| PAN-N/3%Co | 1.79 | 0.003 | 15 |
| PAN-A/10%Co | 25.72 | 0.089 | 13 |
| PAN-N/10%Co | 34.87 | 0.163 | 23 |
| Sample | Rs [Ω/cm2] | Rct [Ω/cm2] | CPE-T | CPE-P |
|---|---|---|---|---|
| PAN-N/1%Co | 53.85 | 326.50 | 6.00 × 10−5 | 0.7798 |
| PAN-N/3%Co | 106.55 | 152.55 | 1.10 × 10−4 | 0.8490 |
| PAN-N/5%Co | 70.10 | 241.50 | 4.15 × 10−5 | 0.7000 |
| PAN-N/7%Co | 70.65 | 406.00 | 1.40 × 10−5 | 0.8604 |
| PAN-N/10%Co | 64.65 | 528.00 | 8.00 × 10−6 | 0.6979 |
| Co3O2 NPs | 58.20 | 52.35 | 6.50 × 10−4 | 0.6905 |
| Co-NC [59] | - | 108.2 | - | - |
| Fe-Co-NCNFs-700 [60] | - | 57.25 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-López, P.; Salatti-Dorado, J.Á.; Rodríguez-Padrón, D.; Cano, M.; Alvarado-Beltrán, C.G.; Puente-Santiago, A.R.; Giner-Casares, J.J.; Luque, R. Mechanochemically Synthetized PAN-Based Co-N-Doped Carbon Materials as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials 2021, 11, 290. https://doi.org/10.3390/nano11020290
Gómez-López P, Salatti-Dorado JÁ, Rodríguez-Padrón D, Cano M, Alvarado-Beltrán CG, Puente-Santiago AR, Giner-Casares JJ, Luque R. Mechanochemically Synthetized PAN-Based Co-N-Doped Carbon Materials as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials. 2021; 11(2):290. https://doi.org/10.3390/nano11020290
Chicago/Turabian StyleGómez-López, Paulette, José Ángel Salatti-Dorado, Daily Rodríguez-Padrón, Manuel Cano, Clemente G. Alvarado-Beltrán, Alain R. Puente-Santiago, Juan J. Giner-Casares, and Rafael Luque. 2021. "Mechanochemically Synthetized PAN-Based Co-N-Doped Carbon Materials as Electrocatalyst for Oxygen Evolution Reaction" Nanomaterials 11, no. 2: 290. https://doi.org/10.3390/nano11020290
APA StyleGómez-López, P., Salatti-Dorado, J. Á., Rodríguez-Padrón, D., Cano, M., Alvarado-Beltrán, C. G., Puente-Santiago, A. R., Giner-Casares, J. J., & Luque, R. (2021). Mechanochemically Synthetized PAN-Based Co-N-Doped Carbon Materials as Electrocatalyst for Oxygen Evolution Reaction. Nanomaterials, 11(2), 290. https://doi.org/10.3390/nano11020290