Surface Properties of 1DTiO2 Microrods Modified with Copper (Cu) and Nanocavities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu-Doped 1D TiO2 MRs
2.3. Methods of Characterization
2.4. Photoelectrochemical Experiments
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Characterization
3.2. Effect of Cu and Temperature on Surface Area (BET) and Porosity (BJH)
3.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Analysis (EDS)
3.3.1. SEM/EDS of Sample Cu/TiO2_500
3.3.2. SEM/EDS of Sample Cu/TiO2_650
3.3.3. SEM/EDS of Sample Cu/TiO2_800
3.3.4. SEM/EDS of Sample Cu/TiO2_950
3.4. High-Resolution Transmission Electron Microscopy (HRTEM) and Selected Area Electron Diffraction (SAED)
3.4.1. HRTEM/SAED of Sample Cu/TiO2_500
3.4.2. HRTEM/SAED of Sample Cu/TiO2_650
3.4.3. HRTEM/SAED of Sample Cu/TiO2_800
3.4.4. HRTEM/SAED of Sample Cu/TiO2_950
3.5. Raman Spectroscopy
3.6. Fourier-Transform Infrared Spectroscopy
3.7. Optical Spectroscopy Results
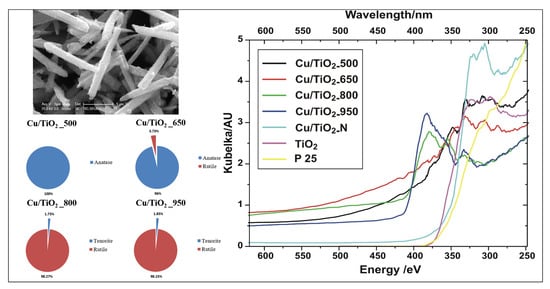
3.7.1. UV-Visible Analysis
3.7.2. PEC Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Klabunde, K.J.; Erickson, L.; Koper, O.; Richards, R. Review of Nanoscale Materials in Chemistry: Environmental Applications. Nanoscale Materials in Chemistry: Environmental Applications. ACS Symp. Ser. 2010, 1045, 1–13. [Google Scholar]
- Negishi, N.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Preparation of Transparent TiO2 Thin Film Photocatalyst and Its Photocatalytic Activity. Chem. Lett. 1995, 9, 841–842. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Pauloze, M.; Varghese, O.K.; Grimes, C.A. Highly Efficient Solar Cells using TiO2 Nanotube Arrays Sensitized with a Donor-Antenna Dye. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Xu, H.; Jia, F.; Ai, Z.; Zhang, L. A General Soft Interface Platform for the Growth and Assembly of Hierarchical Rutile TiO2 Nanorods Spheres. Cryst. Growth Des. 2007, 7, 1216–1219. [Google Scholar] [CrossRef]
- Francioso, L.; Taurino, A.M.; Forleo, A.; Siciliano, P. TiO2 nanowires array fabrication and gas sensing properties. Sens. Actuators B 2008, 130, 70–76. [Google Scholar] [CrossRef]
- Yoong, L.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Byrnea, C.; Moran, L.; Hermosilla, D.; Merayo, N.; Blanco, Á.; Rhatigan, S.; Hinder, S.; Ganguly, P.; Nolan, M.; Pillai, S.C. Effect of Cu doping on the anatase-to-rutile phase transition in TiO2 photocatalysts: Theory and experiments. Appl. Catal. B Environ. 2019, 246, 266–276. [Google Scholar] [CrossRef]
- Colon, G.; Maicu, M.; Hidalgo, M.C.; Navio, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Wong, R.S.; Feng, J.; Hu, X.; Yue, P.L. Discoloration and mineralization of non-biodegradable azo dye orange II by copper-doped TiO2 nanocatalysts. J. Environ. Sci. Health 2004, 39, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly stabilized and finely dispersed Cu2O/TiO2: A promising visible sensitive photocatalyst for continuous production of hydrogen from glycerol: Water mixtures. J. Phys. Chem. 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visiblelight photocatalyst. Ceram. Int. 2015, 41, 8735–8741. [Google Scholar] [CrossRef]
- Nishikiori, H.; Sato, T.; Kubota, S.; Tanaka, N.; Shimizu, Y.; Fujii, T. Preparation of Cudoped TiO2 via refluxing of alkoxide solution and its photocatalytic properties. Res. Chem. Intermed. 2012, 38, 595–613. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaralev, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 11, 2067. [Google Scholar] [CrossRef] [Green Version]
- Obregon, S.; Munoz-Batista, M.; Fernandez-Garcia, M.; Kubacka, A.; Colon, G. Cu/TiO2 system for the photocatalytic H2 production: Influence of structural and surface support features. Appl. Catal. B Environ. 2015, 176, 468–478. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Souier, T.; Liu, S.; Cai, W. Optical, structural and photocatalytic properties of Cu doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Huang, J.; Zhu, C.; Deng, Z.; Shi, H.; Wu, L.; Liu, Z.; Cao, Y. Enhanced hydrogen production by water splitting using Cu doped TiO2 film with prefered (001) orientation. Appl. Surf. Sci. 2014, 292, 161–164. [Google Scholar] [CrossRef]
- Hernández, J.V.; Coste, S.; Murillo, A.G.; Romo, F.C.; Kassiba, A. Effects of Metal Doping (Cu, Ag, Eu) on the Electronic and Optical Behavior of Nanostructured TiO2. J. Alloy. Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Mares, J.; Fajgar, R.; Zenou, V.; Maleckova, M.; Chatzisymeon, E.; Bibova, H.; Jirkovsky, J. The relationship between microstructure and photocatalytic behavior in lanthanum-modified 2D TiO2 nanosheets upon annealing of a freeze-cast precursor. RSC Adv. 2019, 9, 22988–23003. [Google Scholar] [CrossRef] [Green Version]
- Mamon, F.; Fajgar, R.; Jandova, V.; Koci, E.; Jakubec, I.; Zhigunov, A.; Brovdyova, T.; Bakardjieva, S. TiO2 microrods with stacked 3D nanovoids for photoelectrochemical water splitting. Pure Appl. Chem. 2019, 91, 1733–1747. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S. Molybdenum-doped anatase and its extraordinary photocatalytic activity in the degradation of Orange II in the UV and Vis regions. J. Phys. Chem. C 2010, 114, 19308–19317. [Google Scholar] [CrossRef]
- Zenou, V.; Bakardjieva, S. Microstructural analysis of undoped and moderately Sc doped TiO2 anatase nanoparticles using Scherre equation and debye function analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Volfova, L.; Plizingrova, E.; Ecochard, P.; Motlochova, M.; Klementova, M.; Janosikova, P.; Bezdicka, P.; Kupcik, J.; Krysa, J.; Lančok, A.; et al. Tailoring Photocatalytic Activity of TiO2 Nanosheets by 57Fe. J. Phys. Chem. C. 2020, 124, 6669–6682. [Google Scholar] [CrossRef]
- Zenou, V.; Bertolotti, F.; Guagliardi, A.; Toby, B.H.; Dreele, R.V.; Bakardjieva, S. In situ high temperature X ray diffraction study of Sc-doped titanium oxide nanocrystallites. J. Appl. Cryst. 2020, 53, 1452–1461. [Google Scholar] [CrossRef]
- Labar, J.L. Consistent indexing of a (set of) single crystal SAED pattern(s) with the ProcessDiffraction program. Ultramicroscopy 2005, 103, 237–249. [Google Scholar] [CrossRef]
- Colón, G.; Hidalgo, M.C.; Navío, J.A. Effect of ZrO2 incorporation and calcination temperature on the photocatalytic activity of commercial TiO2 for salicylic acid and Cr (VI) photodegradation. Appl. Catal. A Gen. 2002, 231, 185–189. [Google Scholar] [CrossRef]
- Satuf, M.L. Photocatalytic degradation of 4-chlorophenol: A kinetic study. Appl. Catal. B Environ. 2008, 82, 37–49. [Google Scholar] [CrossRef]
- Grätzel, M.; Serpone, N.; Pelizzetti, E. Kinetic studies in heterogeneous photocatalysis. 2. Titania-mediated degradation of 4-chlorophenol alone and in a three-component mixture of 4-chlorophenol, 2,4-Dichlorophenol, and 2,4,5-Trichlorophenol in Air-Equilibrated Aqueous Media. Langmuir 1989, 5, 250–255. [Google Scholar]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Heidelberg/Berlin, Germany, 1912; pp. 387–409. [Google Scholar]
- Ranade, M.R.; Navrotsky, A.; Zhang, H.Z.; Banfield, J.F.; Elder, S.H.; Zaban, A.; Borse, P.H.; Kulkarni, S.K.; Doran, G.S.; Whitfield, H.J. Energetics of nanocrystalline TiO2. PNAS. Proc. Natl. Acad. Sci. USA 2002, 99, 6476–6481. [Google Scholar] [CrossRef] [Green Version]
- Hanaor, D.; Sorrell, C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Tao, J.; Dong, X. Preparation and Photochemical activity of Cr-doped TiO2 nanorods with nanocavities. J. Phys. Chem. C 2010, 114, 2873–2879. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Kumari, M.M.; Srinivas, B.; Kumari, V.D.; Sreedhar, B.; Roddatis, V.; Bondarchuk, O.; Karthik, M.; Neppolian, B.; et al. Cu2O-sensitized TiO2 nanorods with nanocavities for highly efficient photocatalytic hydrogen production under solar irradiation. Sol. Energy Mater. Sol. Cells 2015, 136, 157–166. [Google Scholar] [CrossRef]
- Westen, T.V.; Groot, R. Effect of temperature Cycling on Qswald Ripening. Cryst. Growth Des. 2018, 18, 4952–4962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostwald, W.Z. Biomolecule-assisted synthesis of cobalt sulfide nanowires for application in supercapacitors. J. Phys. Chem. 1900, 34, 495–503. [Google Scholar]
- Aleman, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials. Pure Appl. Chem. 2007, 79, 1801–1827. [Google Scholar] [CrossRef]
- Zhao, Q. Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis. In Micro Nano Technologies, 1st ed.; Elsevier: Liaoning, China, 2020; p. 442. ISBN 9780128147962. [Google Scholar]
- Fu, N.; Wu, Y.; Jin, Z.; Li, G. Structural dependent photoactivities of TiO2 nanoribbon for visible light induced H2 evolution: The role of nanocavities and alternate structutes. Langmuir 2010, 26, 447–455. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Chanda, A.; Vasundhara, M. Significant reduction in the optical band-gap and defect assisted magnetic response in Fe-doped anatase TiO2 nanocrystals as dilute magnetic semiconductors. New J. Chem. 2019, 43, 6048–6062. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Fujii, M. Evidence for Ti Interstitial Induced Extended Visible Absorption and Near Infrared Photoluminescence from Undoped TiO2 Nanoribbons: An In Situ Photoluminescence Study. J. Phys. Chem. C 2013, 117, 23402–23411. [Google Scholar] [CrossRef]
- Kale, G.; Arbuj, S.; Chothe, U.; Khore, S.; Nikam, L.; Kale, B. Highly Crystalline Ordered Cu-doped TiO2 Nanostructure by Paper Templated Method: Hydrogen Production and Dye Degradation under Natural Sunlight. J. Compos. Sci. 2020, 4, 48. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejon, P.; Canto, G.; Mozos, J.L.; Fraxedes, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed Self-Doped Titanium Oxide Thin Films for Efficient Visible-Light Photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Barreca, D.; Fornasiero, P.; Gasparotto, A.; Gombac, V.; Maccato, C.; Montini, T. The potential of supported Cu2O and CuO nanosystems in photocatalytic H2 production. Chemsuschem 2009, 23, 30–33. [Google Scholar]
- Lim, Y.F.; Chua, C.S.; Lee, C.J.J.; Chi, D. Sol-gel deposited Cu2O and CuO thin films for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25928–25934. [Google Scholar] [CrossRef] [PubMed]
- Shaislamov, U.; Lee, H.J. Synthesis and photoelectrochemical properties of a novel CuO/ZnO nanorod photocathode for solar hydrogen generation. J. Korean Phys. Soc. 2016, 69, 1242–1246. [Google Scholar] [CrossRef]
- Kampmann, J.; Betzler, S.; Hajizani, H.; Haringer, S.; Beetz, M.; Harzer, T.; Kraus, J.; Lotsch, B.; Scheu, C.; Pantcheva, R.; et al. How photocorrosion can trick you: A detailed study on lowbandgap Li doped CuO photocathodes for solar hydrogen production. Nanoscale 2020, 12, 7766–7775. [Google Scholar] [CrossRef]
- Bandara, J.; Udawatta, C.; Rajapakse, C. Highly stable CuO incorporated TiO2 catalyst for photocatalytic hydrogen production from H2O. Photochem. Photobiol. Sci. 2005, 4, 857–861. [Google Scholar] [CrossRef]
- Song, K.Y.; Kwon, Y.T.; Choi, G.J.; Lee, W.I. Photocatalytic activity of Cu/TiO2 with the oxidation state of surface-loaded copper. Bull. Korean Chem. Soc. 1999, 20, 957. [Google Scholar]
- Colon, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Fisher, M.B.; Keane, D.A.; Fernandez-Ibanez, P.; Colreavy, J.; Hinder, S.J.; McGuigan, K.G.; Pillai, S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B 2013, 130, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Guo, L.E.Z.; Zhao, D.; Li, X.; Liu, Z. Exposing the photocorrosion mechanism and control strategies of a CuO photocathode. Inorg. Chem. Front. 2019, 6, 2488–2499. [Google Scholar] [CrossRef]

















| Sample | BET [m2g−1] | Average Pore Radius [nm] | Phase | Lattice Parameters [Å] | Crystal Size [nm] | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| TiO2 PDF 21-1272 | - | - | anatase | 3.7852 | 3.7852 | 9.5139 | - |
| TiO2 PDF 21-1276 | - | - | rutile | 4.5933 | 4.5933 | 2.9592 | - |
| CuO PDF 48-1548 | - | - | tenorite | 4.6883 | 3.4229 | 5.1319 | - |
| Cu/TiO2_500 | 108 | 13.2 | anatase | 3.7852 | 3.7852 | 9.5077 | 53 |
| Cu/TiO2_650 | 6.9 | 16.1 | anatase | 3.7841 | 3.7841 | 9.5141 | 94 |
| rutile | 4.5934 | 4.5934 | 2.9593 | 47 | |||
| Cu/TiO2_800 | 3.7 | 25.1 | rutile | 4.5931 | 4.5931 | 2.9592 | 147 |
| tenorite | 4.6898 | 3.4205 | 5.1328 | 122 | |||
| Cu/TiO2_950 | 2.7 | 28.2 | rutile | 4.5931 | 4.5931 | 2.9587 | 157 |
| tenorite | 4.6906 | 3.4193 | 5.1320 | 145 | |||
| Cu/TiO2_500 | Cu/TiO2_650 | ||||
| Chemical Element | Wt [%] | At [%] | Chemical Element | Wt [%] | At [%] |
| O K | 37.3 | 64.2 | O K | 37.2 | 64.1 |
| Ti K | 60.9 | 35.0 | Ti K | 61.2 | 35.2 |
| Cu K | 1.7 | 0.7 | Cu K | 1.6 | 0.7 |
| Cu/TiO2_800 | Cu/TiO2_950 | ||||
| Chemical Element | Wt [%] | At [%] | Chemical Element | Wt [%] | At [%] |
| O K | 37.3 | 64.1 | O K | 35.4 | 62.2 |
| Ti K | 61.2 | 35.2 | Ti K | 63.2 | 37.2 |
| Cu K | 1.5 | 0.7 | Cu K | 1.4 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakardjieva, S.; Mamon, F.; Pinc, Z.; Fajgar, R.; Jakubec, I.; Murafa, N.; Koci, E.; Brovdyova, T.; Lancok, A.; Michna, S.; et al. Surface Properties of 1DTiO2 Microrods Modified with Copper (Cu) and Nanocavities. Nanomaterials 2021, 11, 324. https://doi.org/10.3390/nano11020324
Bakardjieva S, Mamon F, Pinc Z, Fajgar R, Jakubec I, Murafa N, Koci E, Brovdyova T, Lancok A, Michna S, et al. Surface Properties of 1DTiO2 Microrods Modified with Copper (Cu) and Nanocavities. Nanomaterials. 2021; 11(2):324. https://doi.org/10.3390/nano11020324
Chicago/Turabian StyleBakardjieva, Snejana, Filip Mamon, Zdenek Pinc, Radek Fajgar, Ivo Jakubec, Natalija Murafa, Eva Koci, Tatjana Brovdyova, Adriana Lancok, Stefan Michna, and et al. 2021. "Surface Properties of 1DTiO2 Microrods Modified with Copper (Cu) and Nanocavities" Nanomaterials 11, no. 2: 324. https://doi.org/10.3390/nano11020324
APA StyleBakardjieva, S., Mamon, F., Pinc, Z., Fajgar, R., Jakubec, I., Murafa, N., Koci, E., Brovdyova, T., Lancok, A., Michna, S., & Nikolova, R. (2021). Surface Properties of 1DTiO2 Microrods Modified with Copper (Cu) and Nanocavities. Nanomaterials, 11(2), 324. https://doi.org/10.3390/nano11020324