Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocomposite Films
2.3. Electron Beam Irradiation
2.4. Characterization
3. Results and Discussion
3.1. Morphology of HDPE Nanocomposite Films
3.2. ATR–FTIR Spectroscopy
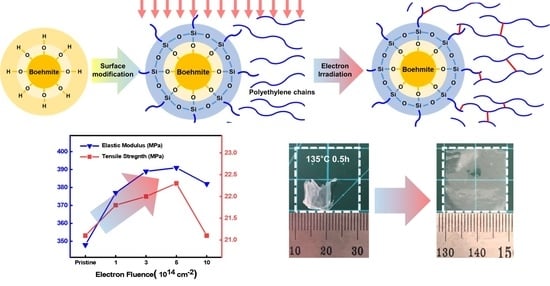
3.3. Mechanical Properties
3.4. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nandi, S.; Bose, S.; Mitra, S.; Ghosh, A.K. Effect of maleic anhydride grafted polyethylene on engineering properties and morphology of fumed silica filled polyethylene blown films. J. Plast. Film Sheeting 2012, 28, 207–227. [Google Scholar] [CrossRef]
- Vázquez-Velázquez, A.R.; Velasco-Soto, M.A.; Pérez-García, S.A.; Licea-Jiménez, L. Functionalization effect on polymer nanocomposite coatings based on TiO2–SiO2 nanoparticles with superhydrophilic properties. Nanomaterials 2018, 8, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barus, S.; Zanetti, M.; Lazzari, M.; Costa, L. Preparation of polymeric hybrid nanocomposites based on PE and nanosilica. Polymer 2009, 50, 2595–2600. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Thai, H.; Mai, D.H.; Tran, H.T.; Vu, M.T. Effect of titanium dioxide on the properties of polyethylene/TiO2 nanocomposites. Compos. Part B Eng. 2013, 45, 1192–1198. [Google Scholar] [CrossRef]
- Gumiero, M.; Peressini, D.; Pizzariello, A.; Sensidoni, A.; Iacumin, L.; Comi, G.; Toniolo, R. Effect of TiO2 photocatalytic activity in a HDPE-based food packaging on the structural and microbiological stability of a short-ripened cheese. Food Chem. 2013, 138, 1633–1640. [Google Scholar] [CrossRef]
- Wang, W.; Li, S. Improvement of dielectric breakdown performance by surface modification in polyethylene/TiO2 nanocomposites. Materials 2019, 12, 3346. [Google Scholar] [CrossRef] [Green Version]
- Ngu, J.; Noshida, I.; Akmil, M.; Chuah, A.L.; Thevy, R.C. Thermal properties of low-density polyethylene/ALPHA-alumina nanocomposites. J. Thermoplast. Compos. Mater. 2012, 25, 415–426. [Google Scholar] [CrossRef]
- Akmil, N.; Luqman, C.; Ahmad, M.; Zaman, K. Improved Mechanical Properties of HDPE/Nano-Alumina Composite through Silane Coupling Agent; AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2012; pp. 500–510. [Google Scholar]
- Liao, C.Z.; Tjong, S.C. Mechanical and thermal performance of high-density polyethylene/alumina nanocomposites. J. Macromol. Sci. Part B 2013, 52, 812–825. [Google Scholar] [CrossRef]
- Ares, A.; Lasagabaster, A.; Abad, M.; Noguerol, R.; Cerecedo, C.; Valcárcel, V.; Caamano, J.; Guitián, F. Effects of silane functionalization of alumina whiskers on high-density polyethylene composites. J. Compos. Mater. 2014, 48, 3141–3151. [Google Scholar] [CrossRef]
- Mohammed, A.S. UHMWPE Nanocomposite Coatings Reinforced with Alumina (Al2O3) Nanoparticles for Tribological Applications. Coatings 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Lins, S.A.B.; Rocha, M.C.G.; d’Almeida, J.R.M. Mechanical and thermal properties of high-density polyethylene/alumina/glass fiber hybrid composites. J. Thermoplast. Compos. Mater. 2019, 32, 1566–1581. [Google Scholar] [CrossRef]
- Saleh, M.; Al-Hajri, Z.; Popelka, A.; Javaid Zaidi, S. Preparation and characterization of alumina HDPE composites. Materials 2020, 13, 250. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-J.; Zha, J.-W.; Wu, Y.-H.; Ren, L.; Dang, Z.-M.; Wu, J. Preparation, microstructure and properties of polyethylene/alumina nanocomposites for HVDC insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3350–3356. [Google Scholar] [CrossRef]
- Ladeira, N.E.; de Melo Furtado, J.G.; Pacheco, E.B. Thermomorphological analysis of Al2O3/HDPE nanocomposites: One approach in function of the processing and vinyltrimethoxysilane (VTMS) content. Polym. Eng. Sci. 2019, 59, 1332–1343. [Google Scholar] [CrossRef]
- Duan, X.; Siew, W.H.; Given, M.; Liggat, J.; He, J. Effect of different surface treatment agents on the physical chemistry and electrical properties of polyethylene nano-alumina nanocomposites. High Volt. 2020, 5, 397–402. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, J.-W.; Yu, S.; Baek, B.K.; Hong, J.P.; Seo, Y.; Kim, W.N.; Hong, S.M.; Koo, C.M. Polyethylene/boron-containing composites for radiation shielding. Thermochim. Acta 2014, 585, 5–9. [Google Scholar] [CrossRef]
- Harrison, C.; Weaver, S.; Bertelsen, C.; Burgett, E.; Hertel, N.; Grulke, E. Polyethylene/boron nitride composites for space radiation shielding. J. Appl. Polym. Sci. 2008, 109, 2529–2538. [Google Scholar] [CrossRef]
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene nanocomposites for power cable insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Nho, Y.-C.; Sohn, J.-Y.; Shin, J.; Park, J.-S.; Lim, Y.-M.; Kang, P.-H. Preparation of nanocomposite γ-Al2O3/polyethylene separator crosslinked by electron beam irradiation for lithium secondary battery. Radiat. Phys. Chem. 2017, 132, 65–70. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. A highly thermostable ceramic-grafted microporous polyethylene separator for safer lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 24119–24126. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Ai, X.; Yang, H.; Cao, Y. TiO2 ceramic-grafted polyethylene separators for enhanced thermostability and electrochemical performance of lithium-ion batteries. J. Membr. Sci. 2016, 504, 97–103. [Google Scholar] [CrossRef]
- Liu, D.; Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.; Gedde, U. Influence of nanoparticle surface treatment on particle dispersion and interfacial adhesion in low-density polyethylene/aluminium oxide nanocomposites. Eur. Polym. J. 2015, 66, 67–77. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Lendvai, L. Polymer/boehmite nanocomposites: A review. J. Appl. Polym. Sci. 2018, 135, 45573. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Datashvili, T.; Huang, B.; Too, J. Tensile properties of LDPE+ boehmite composites. Polym. Compos. 2009, 30, 760–767. [Google Scholar] [CrossRef]
- Brostow, W.; Datashvili, T.; Kao, D.; Too, J. Tribological properties of LDPE+ boehmite composites. Polym. Compos. 2010, 31, 417–425. [Google Scholar] [CrossRef]
- Khumalo, V.; Karger-Kocsis, J.; Thomann, R. Polyethylene/synthetic boehmite alumina nanocomposites: Structure, mechanical, and perforation impact properties. J. Mater. Sci. 2011, 46, 422–428. [Google Scholar] [CrossRef]
- Khumalo, V.M.; Karger-Kocsis, J.; Thomann, R. Polyethylene/synthetic boehmite alumina nanocomposites: Structure, thermal and rheological properties. Express Polym. Lett. 2010, 4, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Pedrazzoli, D. Viscoelastic behaviour and fracture toughness of linear-low-density polyethylene reinforced with synthetic boehmite alumina nanoparticles. eXPRESS Polym. Lett. 2013, 7, 652–666. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Tuba, F.; Khumalo, V.; Pegoretti, A.; Karger-Kocsis, J. Mechanical and rheological response of polypropylene/boehmite nanocomposites. J. Reinf. Plast. Compos. 2014, 33, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Özdilek, C.; Kazimierczak, K.; van der Beek, D.; Picken, S.J. Preparation and properties of polyamide-6-boehmite nanocomposites. Polymer 2004, 45, 5207–5214. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Tamboli, S.; Mhaske, S.; Kale, D. Crosslinked polyethylene. Indian J. Chem. Technol. 2004, 11, 853–864. [Google Scholar]
- Xu, W.; Liu, P.; Li, H.; Xu, X. Effect of electron beam irradiation on mechanical properties of high density polyethylene and its blends with sericite-tridymite-cristobalite. J. Appl. Polym. Sci. 2000, 78, 243–249. [Google Scholar] [CrossRef]
- Sabet, M.; Hassan, A.; Ratnam, C.T. Electron beam irradiation of low-density polyethylene filled with metal hydroxides for wire and cable applications. Polym. Bull. 2012, 69, 1103–1114. [Google Scholar] [CrossRef]
- Seo, D.; Kim, J.; Kang, P.-H.; Seo, C.E.; Lee, J.-H.; Kim, H.-J. Enhancement of flame retardancy and mechanical properties of HDPE/EPM based radiation shielding composites by electron beam irradiation. J. Nucl. Mater. 2012, 429, 99–104. [Google Scholar] [CrossRef]
- Bee, S.-T.; Hassan, A.; Ratnam, C.; Tee, T.-T.; Sin, L.T.; Hui, D. Dispersion and roles of montmorillonite on structural, flammability, thermal and mechanical behaviours of electron beam irradiated flame retarded nanocomposite. Compos. Part B Eng. 2014, 61, 41–48. [Google Scholar] [CrossRef]
- Reiter, G.; Strobl, G.R. Progress in Understanding of Polymer Crystallization; Springer: Berlin, Germany, 2007; Volume 714. [Google Scholar]
- Forster, A.L.; Tsinas, Z.; Al-Sheikhly, M. Effect of irradiation and detection of long-lived polyenyl radicals in highly crystalline ultra-high molar mass polyethylene (UHMMPE) fibers. Polymers 2019, 11, 924. [Google Scholar] [CrossRef] [Green Version]
- Albano, C.; Perera, R.; Silva, P.; Sanchez, Y. Characterization of irradiated PEs/PA6 blends. Polym. Bull. 2006, 57, 901–912. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef]
- Bee, S.-T.; Ratnam, C.; Sin, L.T.; Tee, T.-T.; Hui, D.; Kadhum, A.; Rahmat, A.; Lau, J. Effects of electron beam irradiation on mechanical properties and nanostructural–morphology of montmorillonite added polyvinyl alcohol composite. Compos. Part B Eng. 2014, 63, 141–153. [Google Scholar] [CrossRef]
- Gheysari, D.; Behjat, A.; Haji-Saeid, M. The effect of high-energy electron beam on mechanical and thermal properties of LDPE and HDPE. Eur. Polym. J. 2001, 37, 295–302. [Google Scholar] [CrossRef]
- Badr, Y.; Ali, Z.I.; Zahran, A.H.; Khafagy, R.M. Characterization of gamma irradiated polyethylene films by DSC and X-ray diffraction techniques. Polym. Int. 2000, 49, 1555–1560. [Google Scholar] [CrossRef]
- Sawatari, C.; Nishikido, H.; Matsuo, M. Temperature-dependence of mechanical and morphological properties of ultra-high molecular weight polyethylene cross-linked by electron beam irradiation. Colloid Polym. Sci. 1988, 266, 316–323. [Google Scholar] [CrossRef]
- Lee, S.M.; Jeon, H.-J.; Choi, S.W.; Song, H.H.; Nho, Y.C.; Cho, K. Structure and property modification of bimodal molecular weight distribution polyethylene by electron beam irradiation. Macromol. Res. 2006, 14, 640–645. [Google Scholar] [CrossRef]








| Sample | EB Fluence (×1014 cm−2) | 1st Heating | 2nd Heating | ||||
|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Xc (%) | Tm (°C) | ΔHm (J/g) | Xc (%) | ||
| BA/HDPE | Pristine | 131.9 | 152.92 | 52.1 | 132.9 | 169.61 | 57.8 |
| 5 | 131.1 | 150.81 | 51.4 | 125.3 | 140.96 | 48.1 | |
| 10 | 128.9 | 149.28 | 50.9 | 119.9 | 126.85 | 43.2 | |
| vBA/HDPE | Pristine | 132.1 | 153.2 | 52.2 | 133.3 | 173.35 | 59.1 |
| 5 | 132.6 | 155.5 | 53.1 | 129.8 | 140.64 | 48.0 | |
| 10 | 128.3 | 150.1 | 51.2 | 124.2 | 123.32 | 42.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Jeong, H.Y.; Lee, S.Y.; Cho, S.O. Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film. Nanomaterials 2021, 11, 777. https://doi.org/10.3390/nano11030777
Lee JH, Jeong HY, Lee SY, Cho SO. Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film. Nanomaterials. 2021; 11(3):777. https://doi.org/10.3390/nano11030777
Chicago/Turabian StyleLee, Ju Hyuk, Heon Yong Jeong, Sang Yoon Lee, and Sung Oh Cho. 2021. "Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film" Nanomaterials 11, no. 3: 777. https://doi.org/10.3390/nano11030777
APA StyleLee, J. H., Jeong, H. Y., Lee, S. Y., & Cho, S. O. (2021). Effects of Electron Beam Irradiation on Mechanical and Thermal Shrinkage Properties of Boehmite/HDPE Nanocomposite Film. Nanomaterials, 11(3), 777. https://doi.org/10.3390/nano11030777