The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Manganese Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalyst Characterization
2.3. Catalytic Tests
3. Results
3.1. Structural Properties
3.2. Redox Properties
3.3. In Situ XRD
3.4. Surface Properties
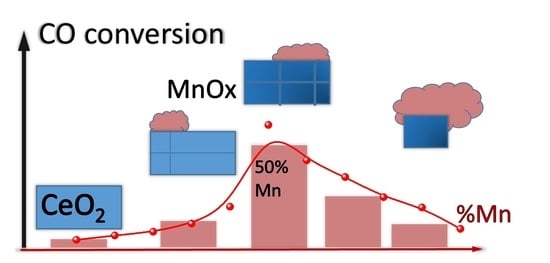
3.5. Catalytic Activity in CO Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, M.S.; Cai, Y.; Yan, Z.; Gath, K.K.; Axnanda, S.; Goodman, D.W. Highly active surfaces for CO oxidation on Rh, Pd, and Pt. Surf. Sci. 2007, 601, 5326–5331. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous Heterogeneous Catalytic Reactions over Mn-Based Oxides for Environmental Applications: A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef] [PubMed]
- Yashnik, S.A.; Ushakov, V.V.; Leonov, N.L.; Ismagilov, Z.R. High-performance Mn-Al-O catalyst on reticulated foam materials for environmentally friendly catalytic combustion. Eurasian Chem. Technol. J. 2015, 17, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fu, P.; Lv, D.; Chen, Y.; Fan, M.; Wu, J.; Meshram, A.; Mu, B.; Li, X.; Xia, Q. Unusual positive effect of SO2 on Mn-Ce mixed-oxide catalyst for the SCR reaction of NOx with NH3. Chem. Eng. J. 2021, 407, 127071. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.C.; Ausen, S.A. Interaction of cerium oxide with noble metals. J. Catal. 1979, 58, 131–143. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Colman-Lerner, E.; Peluso, M.A.; Sambeth, J.; Thomas, H. Cerium, manganese and cerium/manganese ceramic monolithic catalysts. Study of VOCs and PM removal. J. Rare Earths 2016, 34, 675–682. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over MnOx–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2008, 84, 303–312. [Google Scholar] [CrossRef]
- Du, J.; Qu, Z.; Dong, C.; Song, L.; Qin, Y.; Huang, N. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach. Appl. Surf. Sci. 2018, 433, 1025–1035. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Hu, F.; Chen, J.; Zhao, S.; Li, K.; Si, W.; Song, H.; Li, J. Toluene catalytic combustion over copper modified Mn0.5Ce0.5Ox solid solution sponge-like structures. Appl. Catal. A Gen. 2017, 540, 57–67. [Google Scholar] [CrossRef]
- Arena, F.; Di Chio, R.; Fazio, B.; Espro, C.; Spiccia, L.; Palella, A.; Spadaro, L. Probing the functionality of nanostructured MnCeOx catalysts in the carbon monoxide oxidation: Part I. Influence of cerium addition on structure and CO oxidation activity. Appl. Catal. B Environ. 2017, 210, 14–22. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Zhou, Y.; Zhang, L.; Wu, Z.; Yang, S.; Shi, H.; Zhu, Q.; Chen, Y.; Dai, S. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Rao, K.N.; Jampaiah, D.; Reddy, B.M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures. Appl. Catal. B Environ. 2015, 162, 122–132. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Y.; Wu, Z. Low-temperature catalytic oxidation of toluene over mesoporous MnOx–CeO2/TiO2 prepared by sol–gel method. Catal. Commun. 2010, 11, 788–791. [Google Scholar] [CrossRef]
- Liu, X.; Lu, J.; Qian, K.; Huang, W.; Luo, M. A comparative study of formaldehyde and carbon monoxide complete oxidation on MnOx-CeO2 catalysts. J. Rare Earths 2009, 27, 418–424. [Google Scholar] [CrossRef]
- Geng, L.; Chen, B.; Yang, J.; Shui, C.; Ye, S.; Fu, J.; Zhang, N.; Xie, J.; Chen, B. Synergistic effect between Mn and Ce for active and stable catalytic wet air oxidation of phenol over MnCeOx. Appl. Catal. A Gen. 2020, 604, 117774. [Google Scholar] [CrossRef]
- Geng, Y.; Shan, W.; Liu, F.; Yang, S. Adjustment of operation temperature window of Mn-Ce oxide catalyst for the selective catalytic reduction of NOx with NH3. J. Hazard. Mater. 2021, 405, 124223. [Google Scholar] [CrossRef]
- Narayana, B.L.; Mukri, B.D.; Ghosal, P.; Subrahmanyam, C. Mn Ion substituted CeO2 Nano spheres for Low Temperature CO Oxidation: The Promoting Effect of Mn Ions. ChemistrySelect 2016, 1, 3150–3158. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. Intrinsic Activity of MnOx-CeO2 Catalysts in Ethanol Oxidation. Catalysts 2017, 7, 339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jiang, Y.; Gao, J.; Fu, M.; Zou, S.; Li, Y.; Ye, D. Interfaces in MOF-Derived CeO2–MnOX Composites as High-Activity Catalysts for Toluene Oxidation: Monolayer Dispersion Threshold. Catalysts 2020, 10, 681. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Song, Z.; Liu, W.; Zhao, H.; Zhao, M.; Xing, Y.; Ma, Z.A.; Du, H. The catalytic oxidation performance of toluene over the Ce-Mn-Ox catalysts: Effect of synthetic routes. J. Colloid Interface Sci. 2020, 562, 170–181. [Google Scholar] [CrossRef]
- Pankin, I.A.; Kravtsova, A.N.; Polozhentsev, O.E.; Budnyk, A.P.; Tsaturyan, A.A.; Bugaev, A.L.; Trigub, A.L.; Soldatov, A.V. X-ray spectroscopic diagnostics of the structure of quantum dots based on zinc and manganese sulfides and oxides. J. Struct. Chem. 2017, 58, 1633–1640. [Google Scholar] [CrossRef]
- Ni, S.; Tang, X.; Yi, H.; Gao, F.; Wang, C.; Shi, Y.; Zhang, R.; Zhu, W. Novel Mn-Ce bi-oxides loaded on 3D monolithic nickel foam for low-temperature NH3-SCR de-NOx: Preparation optimization and reaction mechanism. J. Rare Earths 2020. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Picasso, G.; Gutiérrez, M.; Pina, M.P.; Herguido, J. Preparation and characterization of Ce-Zr and Ce-Mn based oxides for n-hexane combustion: Application to catalytic membrane reactors. Chem. Eng. J. 2007, 126, 119–130. [Google Scholar] [CrossRef]
- Lin, J.; Guo, Y.; Chen, X.; Li, C.; Lu, S.; Liew, K.M. CO Oxidation over Nanostructured Ceria Supported Bimetallic Cu–Mn Oxides Catalysts: Effect of Cu/Mn Ratio and Calcination Temperature. Catal. Lett. 2018, 148, 181–193. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Characterization and FTIR Studies of MnOx−CeO2 Catalyst for Low-Temperature Selective Catalytic Reduction of NO with NH3. J. Phys. Chem. B 2004, 108, 15738–15747. [Google Scholar] [CrossRef]
- Gao, X.; Dollimore, D. The thermal decomposition of oxalates. Part 26. A kinetic study of the thermal decomposition of manganese(II) oxalate dihydrate. Thermochim. Acta 1993, 215, 47–63. [Google Scholar] [CrossRef]
- Liu, S.; Sun, B.; Zhang, Y.; Li, J.; Resasco, D.E.; Nie, L.; Wang, L. The role of intermediate CoxMn1−xO (x = 0.6–0.85) nanocrystals in the formation of active species for the direct production of lower olefins from syngas. Chem. Commun. 2019, 55, 6595–6598. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Dinse, A.; Aigner, M.; Ulbrich, M.; Johnson, G.R.; Bell, A.T. Effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch Synthesis. J. Catal. 2012, 288, 104–114. [Google Scholar] [CrossRef]
- Paterson, J.; Peacock, M.; Purves, R.; Partington, R.; Sullivan, K.; Sunley, G.; Wilson, J. Manipulation of Fischer-Tropsch Synthesis for Production of Higher Alcohols Using Manganese Promoters. ChemCatChem 2018, 10, 5154–5163. [Google Scholar] [CrossRef]
- Paterson, J.; Partington, R.; Peacock, M.; Sullivan, K.; Wilson, J.; Xu, Z. Elucidating the Role of Bifunctional Cobalt-Manganese Catalyst Interactions for Higher Alcohol Synthesis. Eur. J. Inorg. Chem. 2020, 2020, 2312–2324. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Ma, X.; Mo, S.; Li, S.; Chen, Y. Fabrication of silica supported Mn-Ce benzene oxidation catalyst by a simple and environment-friendly oxalate approach. J. Porous Mater. 2018, 25, 107–117. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Afonasenko, T.N.; Osipov, A.R.; Pochtar, A.A.; Saraev, A.A.; Gerasimov, E.Y. The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Annealing Conditions. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Malakhov, V.V.; Vasilyeva, I.G. Stoichiography and chemical methods of phase analysis of multielement multiphase substances and materials. Russian Chem. Rev. 2008, 77, 351–372. [Google Scholar] [CrossRef]
- CasaXPS: Processing Software for XPS, AES, SIMS and More. Available online: http://www.casaxps.com/ (accessed on 8 January 2020).
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Stobbe, E.R.; De Boer, B.A.; Geus, J.W. The reduction and oxidation behaviour of manganese oxides. Catal. Today 1999, 47, 161–167. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Gerasimov, E.Y.; Afonasenko, T.N. Reduction of double manganese-cobalt oxides: In situ XRD and TPR study. Dalton Trans. 2018, 47, 17153–17159. [Google Scholar] [CrossRef] [PubMed]
- Christel, L.; Pierre, A.; Abel, D.A.M.R. Temperature programmed reduction studies of nickel manganite spinels. Thermochim. Acta 1997, 306, 51–59. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, D.; Wang, Z.; Liu, G.; Liu, H.; Chen, Y. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Mock, S.A.; Sharp, S.E.; Stoner, T.R.; Radetic, M.J.; Zell, E.T.; Wang, R. CeO2 nanorods-supported transition metal catalysts for CO oxidation. J. Colloid Interface Sci. 2016, 466, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trovarelli, A. Catalytic properties of ceria and CeO2-Containing materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Deng, Y.-Q.; Tian, P.; Shang, H.-H.; Xu, J.; Han, Y.-F. Dynamic active sites over binary oxide catalysts: In situ/operando spectroscopic study of low-temperature CO oxidation over MnOx-CeO2 catalysts. Appl. Catal. B Environ. 2016, 191, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Bekheet, M.F.; Grünbacher, M.; Schlicker, L.; Gili, A.; Doran, A.; Epping, J.D.; Gurlo, A.; Klötzer, B.; Penner, S. On the structural stability of crystalline ceria phases in undoped and acceptor-doped ceria materials under in situ reduction conditions. CrystEngComm 2019, 21, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.-S.; Kim, M.-S.; Kim, K.-B. A study on the effect of lithium insertion–extraction on the local structure of lithium manganese oxides using X-ray absorption spectroscopy. J. Power Sources 1999, 81–82, 510–516. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Investigations of the catalytic properties of manganese oxides for the oxygen reduction reaction in alkaline media. J. Electroanal. Chem. 2006, 590, 152–160. [Google Scholar] [CrossRef]
- Ressler, T.; Wong, J.; Roos, J.; Smith, I.L. Quantitative Speciation of Mn-Bearing Particulates Emitted from Autos Burning (Methylcyclopentadienyl)manganese Tricarbonyl-Added Gasolines Using XANES Spectroscopy. Environ. Sci. Technol. 2000, 34, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchert, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P.; et al. Electronic and chemical properties of nanostructured cerium dioxide doped with praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef]
- Christoua, S.Y.; Álvarez-Galvánb, M.C.; Fierrob, J.L.G.; Efstathioua, A.M. Suppression of the oxygen storage and release kinetics in Ce0. 5Zr0. 5O2 induced by P, Ca and Zn chemical poisoning. Appl. Catal. B 2011, 106, 103–113. [Google Scholar]
- Regan, E.; Groutso, T.; Metson, J.B.; Steiner, R.; Ammundsen, B.; Hassell, D.; Pickering, P. Surface and bulk composition of lithium manganese oxides. Surf. Interface Anal. 1999, 27, 1064–1068. [Google Scholar]
- Oku, M.; Hirokawa, K.; Ikeda, S. X-ray photoelectron spectroscopy of manganese—oxygen systems. J. Electron Spectrosc. Relat. Phenom. 1975, 7, 465–473. [Google Scholar] [CrossRef]
- Castro, V.D.; Polzonetti, G. XPS study of MnO oxidation. J. Electron Spectrosc. Relat. Phenom. 1989, 48, 117–123. [Google Scholar] [CrossRef]
- Bondi, J.F.; Oyler, K.D.; Ke, X.; Schiffer, P.; Schaak, R.E. Chemical Synthesis of Air-Stable Manganese Nanoparticles. J. Am. Chem. Soc. 2008, 131, 9144–9145.0445. [Google Scholar] [CrossRef]
- Han, Y.-F.; Chen, F.; Zhong, Z.; Ramesh, K.; Chen, L.; Widjaja, E. Controlled Synthesis, Characterization, and Catalytic Properties of Mn2O3 and Mn3O4 Nanoparticles Supported on Mesoporous Silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef]
- Han, Y.-F.; Chen, L.; Ramesh, K.; Zhong, Z.; Chen, F.; Chin, J.; Mook, H. Coral-like nanostructured a-Mn2O3 nanaocrystals for catalytic combustion of methane Part I. Preparation and characterization. Catal. Today 2008, 131, 35–41. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhang, G.; Zheng, J.; Wang, T.; Liu, X.; Shu, C.; Jiang, L.; Wang, C. Enhanced electrocatalytic performance for methanol oxidation of Pt nanoparticles on Mn3O4-modified multi-walled carbon nanotubes. Int. J. Hydrogen Energy 2012, 37, 11167–11175. [Google Scholar] [CrossRef]
- Ramesh, K.; Chen, L.; Chen, F.; Liu, Y.; Wang, Z.; Han, Y.-F. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts. Catal. Today 2008, 131, 477–482. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Zhan, F. Nitrogen-doped graphene aerogel-supported spinel CoMn2O4 nanoparticles as an efficient catalyst for oxygen reduction reaction. J. Power Sources 2015, 299, 492–500. [Google Scholar] [CrossRef]
- Kong, W.; Gao, B.; Jiang, C.; Chang, A. Influence of the oxygen pressure on the preferred orientation and optical properties of the pulsed-laser deposited Mn1.56Co0.96Ni0.48O4±δ thin films. J. Alloys Compd. 2015, 650, 305–310. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.; Wang, S.; Ding, D.; Chen, M.; Wan, H. Growth and vibrational properties of MnOx thin films on Rh(111). Surf. Sci. 2012, 606, 1507–1511. [Google Scholar] [CrossRef]
- Jadhav, P.R.; Suryawanshi, M.P.; Dalavi, D.S.; Patil, D.S.; Jo, E.A.; Kolekar, S.S.; Wali, A.A.; Karanjkar, M.M.; Kim, J.-H.; Patil, P.S. Design and electro-synthesis of 3-D nanofibers of MnO2 thin films and their application in high performance supercapacitor. Electrochim. Acta 2015, 176, 523–532. [Google Scholar] [CrossRef]
- Kostowskyj, M.A.; Kirk, D.W.; Thorpe, S.J. Ag and Ag–Mn nanowire catalysts for alkaline fuel cells. Int. J. Hydrogen Energy 2010, 35, 5666–5672. [Google Scholar] [CrossRef]
- Hishida, T.; Ohbayashi, K.; Saitoh, T. Hidden relationship between the electrical conductivity and the Mn 2p core-level photoemission spectra in La1−xSrxMnO3. J. Appl. Phys. 2013, 113, 043710. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.-Q.; Meng, M.; Zha, Y.-Q. Surfactant-Assisted Synthesis, Characterizations, and Catalytic Oxidation Mechanisms of the Mesoporous MnOx−CeO2 and Pd/MnOx−CeO2 Catalysts Used for CO and C3H8 Oxidation. J. Phys. Chem. C 2010, 114, 468–477. [Google Scholar] [CrossRef]









| Catalyst | Phase Composition, wt.% | Crystallite Size, nm | Lattice Parameter of Ceria, Å | BET Area, m2/g |
|---|---|---|---|---|
| Mn0Ce1 | 100% CeO2 | 6.0(1) | 5.412(1) | 110 |
| Mn0.1Ce0.9 | 100% CeO2 | 5.9(2) | 5.411(1) | 99 |
| Mn0.15Ce0.85 | 100% CeO2 | 5.5(2) | 5.411(1) | 99 |
| Mn0.25Ce0.75 | 96% CeO2 | 5.5(2) | 5.412(1) | 100 |
| 4% Mn3O4 | 25(3) | |||
| Mn0.45Ce0.55 | 83% CeO2 | 3.5(1) | 5.402(2) | 102 |
| 2% Mn2O3 | - | |||
| 7% Mn3O4 | 25(4) | |||
| 8% Mn5O8 | 10(1) | |||
| Mn0.6Ce0.4 | 72% CeO2 | 4.0(3) | 5.402(1) | 60 |
| 4% Mn2O3 | 22(2) | |||
| 17% Mn3O4 | 25(2) | |||
| 7% Mn5O8 | 13(1) | |||
| Mn0.7Ce0.8 | 62% CeO2 | 4.1(5) | 5.406(1) | 42 |
| 8% Mn2O3 | 20(1) | |||
| 12% Mn3O4 | 25(1) | |||
| 18% Mn5O8 | 14(1) | |||
| Mn0.8Ce0.9 | 45% CeO2 | 5.2(2) | - | 31 |
| 14% Mn2O3 | 20(1) | |||
| 16% Mn3O4 | 25(1) | |||
| 25% Mn5O8 | 13(1) | |||
| Mn0.9Ce0.1 | 45% CeO2 | 5.2(2) | - | 45 |
| 14% Mn2O3 | 20(1) | |||
| 16% Mn3O4 | 25(1) | |||
| 25% Mn5O8 | 13(1) | |||
| Mn1Ce0 | 28% Mn2O3 | 17(1) | - | 32 |
| 34% Mn3O4 | 25(1) | |||
| 38% Mn5O8 | 13(1) |
| Catalyst | Mn2p3/2 | [Mn]/[Mn + Ce] | [Ce3+]/[Ce3++Ce4+], % | ||
|---|---|---|---|---|---|
| Mn2+ 639.9 eV | Mn3+ 640.7 eV | Mn4+ 641.4 eV | |||
| Mn0.1Ce0.9 | 28 | 18 | 54 | 0.08 | 27 |
| Mn0.15Ce0.85 | 28 | 15 | 57 | 0.14 | 28 |
| Mn0.25Ce0.75 | 26 | 16 | 58 | 0.19 | 27 |
| Mn0.45Ce0.55 | 18 | 27 | 55 | 0.41 | 21 |
| Mn0.6Ce0.4 | 16 | 27 | 57 | 0.46 | 22 |
| Mn0.7Ce0.8 | 11 | 22 | 67 | 0.59 | 12 |
| Mn0.8Ce0.9 | 19 | 16 | 65 | 0.61 | 19 |
| Mn0.9Ce0.1 | 18 | 39 | 43 | 0.94 | 17 |
| Mn1Ce0 | 13 | 43 | 44 | 1.00 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulavchenko, O.A.; Afonasenko, T.N.; Osipov, A.R.; Pochtar’, A.A.; Saraev, A.A.; Vinokurov, Z.S.; Gerasimov, E.Y.; Tsybulya, S.V. The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Manganese Content. Nanomaterials 2021, 11, 988. https://doi.org/10.3390/nano11040988
Bulavchenko OA, Afonasenko TN, Osipov AR, Pochtar’ AA, Saraev AA, Vinokurov ZS, Gerasimov EY, Tsybulya SV. The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Manganese Content. Nanomaterials. 2021; 11(4):988. https://doi.org/10.3390/nano11040988
Chicago/Turabian StyleBulavchenko, Olga A., Tatyana N. Afonasenko, Alexey R. Osipov, Alena A. Pochtar’, Andrey A. Saraev, Zahar S. Vinokurov, Evgeny Yu. Gerasimov, and Sergey V. Tsybulya. 2021. "The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Manganese Content" Nanomaterials 11, no. 4: 988. https://doi.org/10.3390/nano11040988
APA StyleBulavchenko, O. A., Afonasenko, T. N., Osipov, A. R., Pochtar’, A. A., Saraev, A. A., Vinokurov, Z. S., Gerasimov, E. Y., & Tsybulya, S. V. (2021). The Formation of Mn-Ce Oxide Catalysts for CO Oxidation by Oxalate Route: The Role of Manganese Content. Nanomaterials, 11(4), 988. https://doi.org/10.3390/nano11040988