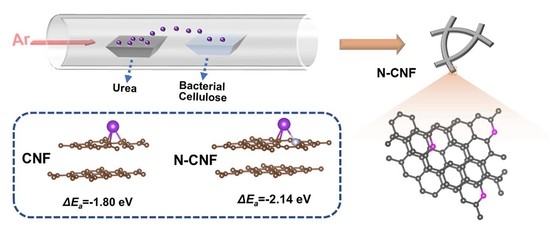
Ultra-Stable Potassium Ion Storage of Nitrogen-Doped Carbon Nanofiber Derived from Bacterial Cellulose
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
2.3. Computational Methods
2.4. Electrochemical Measurement
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-Dimensional Black Phosphorus Nanomaterials: Emerging Advances in Electrochemical Energy Storage Science. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.-C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Li, L.; Zhao, R.; Xu, T.; Wang, D.; Pan, D.; Zhang, K.; Yu, C.; Lu, X.; He, G.; Bai, Y. Stabilizing a high-voltage LiNi0.5Mn1.5O4 cathode towards all solid state batteries: A Li–Al–Ti–P–O solid electrolyte nano-shell with a host material. Nanoscale 2019, 11, 8967–8977. [Google Scholar] [CrossRef]
- Zhou, N.F.; Qin, W.; Wu, C.; Jia, C.K. Graphene-attached vanadium sulfide composite prepared via microwave-assisted hydro-thermal method for high performance lithium ion batteries. J. Alloys Compd. 2020, 834, 155073. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Y.; Zhuang, N.; Li, X.; Yuan, X.; Li, J.; Hong, G.; Mai, W. High-concentration ether-based electrolyte boosts the electrochemical performance of SnS2–reduced graphene oxide for K-ion batteries. J. Mater. Chem. A 2019, 7, 19332–19341. [Google Scholar] [CrossRef]
- Zu, L.; Zhang, W.; Qu, L.; Liu, L.; Li, W.; Yu, A.; Zhao, D. Mesoporous Materials for Electrochemical Energy Storage and Conversion. Adv. Energy Mater. 2020, 10, 2002152. [Google Scholar] [CrossRef]
- Li, J.L.; Zhuang, N.; Xie, J.P.; Li, X.D.; Zhuo, W.C.; Wang, H.; Na, J.B.; Li, X.B.; Yamauchi, Y.; Mai, W.J. K-ion storage enhancement in Sb2O3/reduced graphene oxide using ether-based electrolyte. Adv. Energy Mater. 2020, 10, 1903455. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.G.; Ji, X.B.; Jia, C.K.; Wang, H.Y. Advancements and challenges in potassium ion batteries: A comprehen-sive review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Ma, L.; Yu, C.; Ji, Z.; Pan, L.; Mai, W. Graphite Anode for Potassium ion batteries: Current Status and Perspective. Energy Environ. Mater. 2021. [Google Scholar] [CrossRef]
- Zhang, E.J.; Jia, X.N.; Wang, B.; Wang, J.; Yu, X.Z.; Lu, B.G. Carbon dots@rGO paper as freestanding and flexible potassium-ion batteries anode. Adv. Sci. 2020, 7, 2000470. [Google Scholar] [CrossRef]
- Cheng, X.L.; Li, D.J.; Wu, Y.; Xu, R.; Yu, Y. Bismuth nanospheres embedded in three-dimensional (3D) porous graphene frame-works as high performance anodes for sodium- and potassium-ion batteries. J. Mater. Chem. A 2019, 7, 4913–4921. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, T.; Peng, L.; Yao, W.; Kang, F.; Shu, J.; Yang, C. A compact Bi2WO6 microflowers anode for potassium-ion storage: Taming a sequential phase evolution toward stable electrochemical cycling. Nano Energy 2021, 82, 105784. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Lai, H.; Zhao, Z.; Li, J.; Zhang, W.; Xie, W.; Liu, Y.; Mai, W. A Robust Solid Electrolyte Interphase Layer Augments the Ion Storage Capacity of Bimetallic-Sulfide-Containing Potassium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 14740–14747. [Google Scholar] [CrossRef]
- Li, J.; Qin, W.; Xie, J.; Lei, H.; Zhu, Y.; Huang, W.; Xu, X.; Zhao, Z.; Mai, W. Sulphur-doped reduced graphene oxide sponges as high-performance free-standing anodes for K-ion storage. Nano Energy 2018, 53, 415–424. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.L.; Xing, Z.; Lam, C.W.K.; Pang, S.S.; Zhang, W.; Ju, Z.C. Advanced carbon-based anodes for potassium-ion bat-teries. Adv Energy Mater. 2019, 9, 1900343. [Google Scholar] [CrossRef]
- Cui, R.C.; Xu, B.; Dong, H.J.; Yang, C.C.; Jiang, Q. N/O Dual-doped environment-friendly hard carbon as advanced anode for potassium-ion batteries. Adv. Sci. 2020, 7, 1902547. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ma, R.; Zhang, Q.; Jia, X.; Lu, B. Graphite Anode for a Potassium-Ion Battery with Unprecedented Performance. Angew. Chem. Int. Ed. 2019, 58, 10500–10505. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Ozdemir, B.; Bao, W.; Chen, Y.; Dai, J.; Lin, H.; Xu, Y.; Gu, F.; Barone, V.; et al. Potassium Ion Batteries with Graphitic Materials. Nano Lett. 2015, 15, 7671–7677. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, N.; Xie, J.; Zhu, Y.; Lai, H.; Qin, W.; Javed, M.S.; Xie, W.; Mai, W. Carboxymethyl Cellulose Binder Greatly Stabilizes Porous Hollow Carbon Submicrospheres in Capacitive K-Ion Storage. ACS Appl. Mater. Interfaces 2019, 11, 15581–15590. [Google Scholar] [CrossRef]
- Lin, X.; Huang, J.; Zhang, B. Correlation between the microstructure of carbon materials and their potassium ion storage performance. Carbon 2019, 143, 138–146. [Google Scholar] [CrossRef]
- Alvin, S.; Chandra, C.; Kim, J. Controlling intercalation sites of hard carbon for enhancing Na and K storage performance. Chem. Eng. J. 2021, 411, 128490. [Google Scholar] [CrossRef]
- Bobyleva, Z.V.; Drozhzhin, O.A.; Dosaev, K.A.; Kamiyama, A.; Ryazantsev, S.V.; Komaba, S.; Antipov, E.V. Unveiling pseudo-capacitive behavior of hard carbon anode materials for sodium-ion batteries. Electrochim. Acta 2020, 354, 136647. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Górka, J.; Simone, V.; Simonin, L.; Martinet, S.; Vix-Guterl, C. Insights on the Na+ ion storage mechanism in hard carbon: Discrimination between the porosity, surface functional groups and defects. Nano Energy 2018, 44, 327–335. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Huang, J.; Zou, J.; Chen, S.; Cheng, H.; Jiang, C.; Gao, P.; Niu, X. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochim. Acta 2019, 306, 446–453. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mecha-nism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Xu, B.; Qi, S.; Li, F.; Peng, X.; Cai, J.; Liang, J.; Ma, J. Cotton-derived oxygen/sulfur co-doped hard carbon as advanced anode material for potassium-ion batteries. Chin. Chem. Lett. 2020, 31, 217–222. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Passerini, S. Non-aqueous potassium-ion batteries: A review. Curr. Opin. Electrochem. 2018, 9, 41–48. [Google Scholar] [CrossRef]
- Song, P.; Shen, X.; He, X.; Feng, K.; Kong, L.; Ji, Z.; Zhai, L.; Zhu, G.; Zhang, D. Cellulose-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitors. Cellulose 2018, 26, 1195–1208. [Google Scholar] [CrossRef]
- Ding, C.; Liu, T.; Yan, X.; Huang, L.; Ryu, S.; Lan, J.; Yu, Y.; Zhong, W.-H.; Yang, X. An Ultra-microporous Carbon Material Boosting Integrated Capacitance for Cellulose-Based Supercapacitors. Nano-Micro Lett. 2020, 12, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Belaustegui, Y.; Pantò, F.; Urbina, L.; Corcuera, M.A.; Eceiza, A.; Palella, A.; Triolo, C.; Santangelo, S. Bacterial-cellulose-derived carbonaceous electrode materials for water desalination via capacitive method: The crucial role of defect sites. Desalination 2020, 492, 114596. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Xu, H.; Zhang, L. Enhanced capacitive deionization by nitrogen-doped porous carbon nanofiber aerogel derived from bacterial-cellulose. J. Electroanal. Chem. 2018, 822, 81–88. [Google Scholar] [CrossRef]
- Illa, M.P.; Khandelwal, M.; Sharma, C.S. Bacterial cellulose-derived carbon nanofibers as anode for lithium-ion batteries. Emer-gent Mater. 2018, 1, 105–120. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yu, Y. A facile strategy toward sodium-ion batteries with ultra-long cycle life and high initial Coulombic effi-ciency: Free-standing porous carbon nanofiber film derived from bacterial cellulose. Energy Storage Mater. 2019, 22, 105–112. [Google Scholar] [CrossRef]
- Zhang, J.M.; Hua, Q.; Li, J.; Yuan, J.; Peijs, T.; Dai, Z.; Zhang, Y.; Zheng, Z.; Zheng, L.; Tang, J. Cellulose-Derived Highly Porous Three-Dimensional Activated Carbons for Supercapacitors. ACS Omega 2018, 3, 14933–14941. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Z.; Zhang, Q.; Natan, A.; Yang, Y.; Cao, D.; Zhu, H. Bacterial-Derived, Compressible, and Hierarchical Porous Carbon for High-Performance Potassium-Ion Batteries. Nano Lett. 2018, 18, 7407–7413. [Google Scholar] [CrossRef]
- Hu, J.X.; Xie, Y.Y.; Yin, M.; Zhang, Z.A. Nitrogen doping and graphitization tuning coupled hard carbon for superior potassi-um-ion storage. J. Energy Chem. 2020, 49, 327–334. [Google Scholar] [CrossRef]
- Wang, G.; Yu, L.; Gao, J.; Li, Y.; Zeng, S.; Zhang, G. Dual-functional template-induced in situ polymerization process enables the hierarchical carbonaceous nanotubes with simultaneous Sn cluster incorporation and nitrogen-doping for superior potas-sium-ion storage. ACS Appl. Mater. Interfaces 2021, 13, 13139–13148. [Google Scholar]
- Xiong, P.; Zhao, X.; Xu, Y. Nitrogen-Doped Carbon Nanotubes Derived from Metal-Organic Frameworks for Potassium-Ion Battery Anodes. ChemSusChem 2018, 11, 202–208. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Zhang, T.; Wang, B.; Wang, Y.; Wang, L.; Wei, J. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation. Energy 2020, 211, 118561. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Duan, H.; Xu, A.; Xia, Q.; Yan, Y.; Wu, S. N-doped carbon nanofibers with internal cross-linked multiple pores for both ultra-long cycling life and high capacity in highly durable K-ion battery anodes. Electrochim. Acta 2020, 337, 135767. [Google Scholar] [CrossRef]
- Jin, Q.Z.; Wang, K.L.; Feng, P.Y.; Zhang, Z.C.; Cheng, S.J.; Jiang, K. Surface-dominated storage of heteroatoms-doping hard car-bon for sodium-ion batteries. Energy Storage Mater. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Li, J.; Lei, H.; Zhuo, W.; Qin, W.; Cai, X.; Hui, K.N.; Pan, L.; Mai, W. Insights on the mechanism of Na-ion storage in expanded graphite anode. J. Energy Chem. 2021, 53, 56–62. [Google Scholar] [CrossRef]
- Li, Y.; Ni, B.; Li, X.; Wang, X.; Zhang, D.; Zhao, Q.; Li, J.; Lu, T.; Mai, W.; Pan, L. High-Performance Na-Ion Storage of S-Doped Porous Carbon Derived from Conjugated Microporous Polymers. Nano-Micro Lett. 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; He, Q.; Xiong, F.; Zhou, J.; Zhao, Y.; Mai, L.; Zhang, L. Active sites enriched hard carbon porous nanobelts for stable and high-capacity potassium-ion storage. Nano Energy 2020, 77, 105018. [Google Scholar] [CrossRef]
- Luo, C.; Shea, J.J.; Huang, J. A carboxylate group-based organic anode for sustainable and stable sodium ion batteries. J. Power Source 2020, 453, 227904. [Google Scholar] [CrossRef]
- Fernandez-Escamilla, H.N.; Guerrero-Sanchez, J.; Contreras, E.; Ruiz-Marizcal, J.M.; Alonso-Nunez, G.; Contreras, O.E.; Felix-Navarro, R.M.; Romo-Herrera, J.M.; Takeuchi, N. Understanding the Selectivity of the Oxygen Reduction Reaction at the Atomistic Level on Nitrogen-Doped Graphitic Carbon Materials. Adv. Energy Mater. 2020, 11, 2002459. [Google Scholar] [CrossRef]
- Yang, J.; He, W.; Jiang, Q.; Chen, Z.; Ju, H.; Xue, X.; Xu, Z.; Hu, P.; Yu, G. Hydrogen-dominated metal-free growth of graphit-ic-nitrogen doped graphene with n-type transport behaviors. Carbon 2020, 161, 123–131. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Zheng, Y.; Xia, B.; Zhou, X.; Xu, H.; Qiao, S. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. [Google Scholar] [CrossRef]
- Liu, S.S.; Deng, C.W.; Yao, L.; Zhong, H.X.; Zhang, H.M. The key role of metal dopants in nitrogen-doped carbon xerogel for ox-ygen reduction reaction. J. Power Source 2014, 269, 225–235. [Google Scholar] [CrossRef]
- Xie, J.P.; Zhu, Y.Q.; Zhuang, N.; Lei, H.; Zhu, W.L.; Fu, Y.; Javed, M.S.; Li, J.L.; Mai, W.J. Rational design of metal organic frame-work-derived FeS2 hollow nanocages@reduced graphene oxide for K-ion storage. Nanoscale 2018, 10, 17092–17098. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, J.L.; Gong, Z.W.; Xie, J.P.; Lu, T.; Pan, L.K. Nitrogen and sulfur co-doped vanadium carbide MXene for highly re-versible lithium-ion storage. J. Colloid Interf. Sci. 2021, 587, 489–498. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Pan, L.; Li, J.; Wang, C.; Wang, G. Facile self-templating synthesis of layered carbon with N, S dual doping for highly efficient sodium storage. Carbon 2021, 173, 31–40. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Ahmed, S.M.; Suo, G.; Wang, W.A.; Feng, L.; Hou, X.; Yang, Y.; Ye, X.; Zhang, L. Amorphous carbon coated SnO2 nanohseets on hard carbon hollow spheres to boost potassium storage with high surface capacitive contributions. J. Colloid Interface Sci. 2020, 574, 174–181. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Li, N.; Han, X.; Luo, X.; Xie, K.Y.; Wei, B.Q.; Xia, Z.H. Design principles of pseudocapacitive carbon anode mate-rials for ultrafast sodium and potassium-ion batteries. J. Mater. Chem. A 2020, 8, 7756–7764. [Google Scholar] [CrossRef]
- Adekoya, D.; Li, M.; Hankel, M.; Lai, C.; Balogun, M.S.; Tong, Y.X.; Zhang, S.Q. Design of a 1D/2D C3N4/rGO composite as an an-ode material for stable and effective potassium storage. Energy Storage Mater. 2020, 25, 495–501. [Google Scholar] [CrossRef]
- Tan, J.C.; Li, D.; Liu, Y.Q.; Zhang, P.; Qu, Z.H.; Yan, Y.; Hu, H.; Cheng, H.Y.; Zhang, J.X.; Dong, M.Y.; et al. A self-supported 3D aerogel network lithium-sulfur battery cathode: Sulfur spheres wrapped with phospho-rus doped graphene and bridged with carbon nanofibers. J. Mater. Chem. A 2020, 8, 7980–7990. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Li, X.; Lei, H.; Zhuo, W.; Li, X.; Hong, G.; Hui, K.N.; Pan, L.; Mai, W. Ultrahigh “Relative Energy Density” and Mass Loading of Carbon Cloth Anodes for K-Ion Batteries. CCS Chem. 2021, 3, 791–799. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, J.; Li, Z.; Ji, Y.; Mai, W.; Wang, H. Ultra-Stable Potassium Ion Storage of Nitrogen-Doped Carbon Nanofiber Derived from Bacterial Cellulose. Nanomaterials 2021, 11, 1130. https://doi.org/10.3390/nano11051130
Ma L, Li J, Li Z, Ji Y, Mai W, Wang H. Ultra-Stable Potassium Ion Storage of Nitrogen-Doped Carbon Nanofiber Derived from Bacterial Cellulose. Nanomaterials. 2021; 11(5):1130. https://doi.org/10.3390/nano11051130
Chicago/Turabian StyleMa, Liang, Jinliang Li, Zhibin Li, Yingying Ji, Wenjie Mai, and Hao Wang. 2021. "Ultra-Stable Potassium Ion Storage of Nitrogen-Doped Carbon Nanofiber Derived from Bacterial Cellulose" Nanomaterials 11, no. 5: 1130. https://doi.org/10.3390/nano11051130
APA StyleMa, L., Li, J., Li, Z., Ji, Y., Mai, W., & Wang, H. (2021). Ultra-Stable Potassium Ion Storage of Nitrogen-Doped Carbon Nanofiber Derived from Bacterial Cellulose. Nanomaterials, 11(5), 1130. https://doi.org/10.3390/nano11051130