Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Material Characterizations
3. Results
3.1. Rheological Characterization of the Blend Components and Blends
3.2. SWCNT Macrodispersion in Blends Characterized by Light Microscopy
3.3. Blend Morphology Characterized by Scanning Electron Microscopy (Constant SWCNT Content of 3 wt.%)
3.4. CNT Localization in the Blends
3.5. Thermal Conductivity of Composite and Blend Material
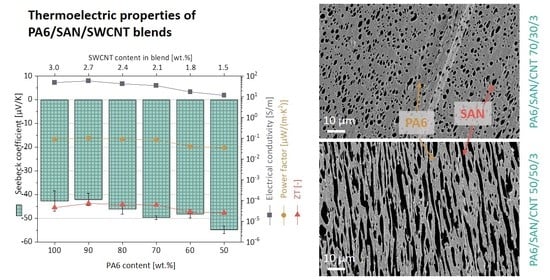
3.6. Thermoelectric Measurements on the Blend Composites
3.6.1. Blends with 3 wt.% SWCNT Content
3.6.2. Blends with Constant SWCNT Content of 3 wt.% in the PA6 Component
3.6.3. Blends with 50/50 wt.% and Low SWCNT Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Material | Surface Tension Total γ [mN/m] | Surface Tension Disperse Part γd [mN/m] | Surface Tension Polar Part γP [mN/m] | Temperature Coefficient dγ/dT [mN/m·°C] | Polarity [%] |
|---|---|---|---|---|---|
| PA6 | 36.1 @ 265 °C [80] | −0.065 [81] | 28 [81] | ||
| PA6 | 37.7 @ 240 °C [75] | 27.2 [75] | 10.6 [75] | 28 * | |
| SAN 1 [76] | 45.3 @ 23 °C 33.1 @ 180 °C | 36.8 @ 23 °C | 8.5 @ 23 °C | −0.078 * | 19 * |
| SAN 2 | 29.0 @ 270 °C, 30.0 @ 250 °C [77] | 24 [68] | |||
| PMMA 1 [76] | 40.5 @ 23 °C 29.9 @ 180 °C | 32.4 @ 23 °C | 8.1 @ 23 °C | −0.067 * | 20 * |
| PMMA 2 | 28.9 @ 180 °C [78] | −0.076 [78] | 28 [82] | ||
| MWCNT 1 [73] | 45.3 | 18.4 | 26.9 | 59 * | |
| MWCNT 2 [74] | 27.8 | 17.6 | 10.2 | 37 * |
| PA6/SAN/SWCNT | Volume Conductivity [S/m] | Seebeck Coefficient [µV/K] | Power Factor [µW/m·K2] | ZT [−] |
|---|---|---|---|---|
| 100/0/6 | 71.6 | −40.2 ± 0.4 | 0.1157 | 3.9 × 10−5 1 |
| 100/0/3 | 49.3 | −42.7 ± 4.3 | 0.0899 | 4.6 × 10−5 2 |
| 90/10/3 | 67.1 | −53.0 ± 5.3 | 0.1884 | 1.3 × 10−4 3 |
| 80/20/3 | 44.6 | −48.5 ± 0.7 | 0.1049 | 7.3 × 10−5 3 |
| 70/30/3 | 88.0 | −50.0 ± 2.1 | 0.2165 | 1.5 × 10−4 3 |
| 60/40/3 | 42.1 | −51.7 ± 1.6 | 0.1127 | 7.9 × 10−5 3 |
| 50/50/3 | 29.5 | −50.6 ± 2.3 | 0.0757 | 5.3 × 10−5 3 |
| 90/10/2.7 | 58.5 | −42.1 ± 2.6 | 0.1035 | 7.2 × 10−5 3 |
| 80/20/2.4 | 42.9 | −49.1 ± 2.1 | 0.0912 | 6.4 × 10−5 3 |
| 70/30/2.1 | 35.1 | −49.7 ± 0.8 | 0.0867 | 6.1 × 10−5 3 |
| 60/40/1.8 | 17.4 | −48.1 ± 1.7 | 0.0402 | 2.8 × 10−5 3 |
| 50/50/1.5 | 11.8 | −54.8 ± 1.6 | 0.0356 | 2.5 × 10−5 3 |
| 50/50/1 | 4.8 | −36.7 ± 0.8 | 0.0065 | 4.6 × 10−6 3 |
| 50/50/0.5 | 1.2 | −25.8 ± 1.2 | 0.0008 | 5.7 × 10−7 3 |
| 50/50/0.25 | 0.1 | −11.4 ± 0.2 | 1.1 × 10−5 | 7.8 × 10−9 3 |
| PA6/PMMA/SWCNT | Volume Conductivity [S/m] | Seebeck Coefficient [µV/K] | Power Factor [µW/m·K2] | ZT [−] |
|---|---|---|---|---|
| 100/0/3 | 49.3 | −42.7 ± 4.3 | 0.0899 | 4.6 × 10−5 1 |
| 90/10/3 | 79.2 | −41.2 ± 2.0 | 0.1347 | 8.9 × 10−4 2 |
| 80/20/3 | 42.2 | −48.0 ± 2.7 | 0.0973 | 6.4 × 10−5 2 |
| 70/30/3 | 39.6 | −48.5 ± 0.5 | 0.0931 | 6.1 × 10−4 2 |
| 60/40/3 | 65.2 | −44.7 ± 2.2 | 0.1304 | 8.6 × 10−5 2 |
| 50/50/3 | 43.4 | −49.3 ± 0.2 | 0.1056 | 7.0 × 10−5 2 |
| 90/10/2.7 | 39.4 | −47.7 ± 3.2 | 0.0898 | 5.9 × 10−5 2 |
| 80/20/2.4 | 38.2 | −46.8 ± 4.1 | 0.0836 | 5.5 × 10−5 2 |
| 70/30/2.1 | 33.2 | −50.7 ± 1.0 | 0.0853 | 5.6 × 10−5 2 |
| 60/40/1.8 | 25.3 | −51.1 ± 1.2 | 0.0662 | 4.4 × 10−5 2 |
| 50/50/1.5 | 17.7 | −49.6 ± 0.7 | 0.0436 | 2.9 × 10−5 2 |
| 50/50/1 | 4.2 | −30.3 ± 0.4 | 0.0038 | 2.5 × 10−6 2 |
| 50/50/0.5 | 0.9 | −25.9 ± 0.8 | 0.0006 | 3.9 × 10−7 2 |
| 50/50/0.25 | 0.2 | −25.7 ± 0.4 | 0.0002 | 7.7 × 10−8 2 |
References
- A steady approach. Nat. Mater. 2021, 20, 437. [CrossRef]
- Babu, C.; Ponnambalam, P. The role of thermoelectric generators in the hybrid PV/T systems: A review. Energy Convers. Manag. 2017, 151, 368–385. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.-G.; Zou, J. Chapter 1—High-Performance Thermoelectric Materials for Solar Energy Application. In Emerging Materials for Energy Conversion and Storage; Cheong, K.Y., Impellizzeri, G., Fraga, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–38. [Google Scholar] [CrossRef]
- Kamarudin, M.A.; Sahamir, S.R.; Datta, R.S.; Long, B.D.; Mohd Sabri, M.F.; Mohd Said, S. A Review on the Fabrication of Polymer-Based Thermoelectric Materials and Fabrication Methods. Sci. World J. 2013, 2013, 713640. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Song, Y.; Hsu, J.-H.; Yu, C.; Stevens, D.L.; Grunlan, J.C. Organic thermoelectric thin films with large p-type and n-type power factor. J. Mater. Sci. 2021, 56, 4291–4304. [Google Scholar] [CrossRef]
- Zoui, M.A.; Bentouba, S.; Stocholm, J.G.; Bourouis, M. A Review on Thermoelectric Generators: Progress and Applications. Energies 2020, 13, 3606. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Park, M.; Park, S.-J. Recent Advances in Organic Thermoelectric Materials: Principle Mechanisms and Emerging Carbon-Based Green Energy Materials. Polymers 2019, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429. [Google Scholar] [CrossRef]
- Yao, C.-J.; Zhang, H.-L.; Zhang, Q. Recent Progress in Thermoelectric Materials Based on Conjugated Polymers. Polymers 2019, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef]
- Brun, J.-F.; Binet, C.; Tahon, J.-F.; Addad, A.; Tranchard, P.; Barrau, S. Thermoelectric properties of bulk multi-walled carbon nanotube-poly(vinylidene fluoride) nanocomposites: Study of the structure/property relationships. Synth. Met. 2020, 269, 116525. [Google Scholar] [CrossRef]
- Montgomery, D.S.; Hewitt, C.A.; Barbalace, R.; Jones, T.; L. Carroll, D. Spray Doping Method to Create a Low-Profile High-Density Carbon Nanotube Thermoelectric Generator. Carbon 2016, 96, 778–781. [Google Scholar] [CrossRef]
- Freeman, D.D.; Choi, K.; Yu, C. N-Type Thermoelectric Performance of Functionalized Carbon Nanotube-Filled Polymer Composites. PLoS ONE 2012, 7, e47822. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Terakita, D.; Tseng, A.C.; Naguib, H.E. Study on the thermoelectric properties of PVDF/MWCNT and PVDF/GNP composite foam. Smart Mater. Struct. 2015, 24, 085034. [Google Scholar] [CrossRef] [Green Version]
- Antar, Z.; Feller, J.F.; Noël, H.; Glouannec, P.; Elleuch, K. Thermoelectric behaviour of melt processed carbon nanotube/graphite/poly(lactic acid) conductive biopolymer nanocomposites (CPC). Mater. Lett. 2012, 67, 210–214. [Google Scholar] [CrossRef]
- Liebscher, M.; Gärtner, T.; Tzounis, L.; Mičušík, M.; Pötschke, P.; Stamm, M.; Heinrich, G.; Voit, B. Influence of the MWCNT surface functionalization on the thermoelectric properties of melt-mixed polycarbonate composites. Compos. Sci. Technol. 2014, 101, 133–138. [Google Scholar] [CrossRef]
- Tzounis, L.; Petousis, M.; Grammatikos, S.; Vidakis, N. 3D Printed Thermoelectric Polyurethane/Multiwalled Carbon Nanotube Nanocomposites: A Novel Approach towards the Fabrication of Flexible and Stretchable Organic Thermoelectrics. Materials 2020, 13, 2879. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. (Eds.) Carbon Nanotubes, 1st ed.; Elsevier Science Limited: Oxford, UK, 1996. [Google Scholar]
- Hung, N.T.; Nugraha, A.R.T.; Saito, R. Thermoelectric Properties of Carbon Nanotubes. Energies 2019, 12, 4561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Q.; Chen, G. Carbon and carbon composites for thermoelectric applications. Carbon Energy 2020, 2, 408–436. [Google Scholar] [CrossRef]
- Romero, H.E.; Sumanasekera, G.U.; Mahan, G.D.; Eklund, P.C. Thermoelectric power of single-walled carbon nanotube films. Phys. Rev. B 2002, 65, 205410. [Google Scholar] [CrossRef] [Green Version]
- Mytafides, C.K.; Tzounis, L.; Karalis, G.; Formanek, P.; Paipetis, A.S. High-Power All-Carbon Fully Printed and Wearable SWCNT-Based Organic Thermoelectric Generator. ACS Appl. Mater. Interfaces 2021, 13, 11151–11165. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.-G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.-L.; Yang, Y.-L.; Chen, Z.-G. Flexible thermoelectric materials and devices: From materials to applications. Materialstoday 2021, in press. [Google Scholar] [CrossRef]
- Shi, X.-L.; Chen, W.-Y.; Zhang, T.; Zou, J.; Chen, Z.-G. Fiber-based thermoelectrics for solid, portable, and wearable electronics. Energy Environ. Sci. 2021, 14, 729–764. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, L.; Zhang, W.; Liufu, S.; Chen, X. Enhanced Thermoelectric Performance of Single-Walled Carbon Nanotubes/Polyaniline Hybrid Nanocomposites. ACS Nano 2010, 4, 2445–2451. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Fan, S. A Promising Approach to Enhanced Thermoelectric Properties Using Carbon Nanotube Networks. Adv. Mater. 2010, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Stevens, B.; Hsu, J.-H.; Bureau, R.; Hagen, D.A.; Regev, O.; Yu, C.; Grunlan, J.C. Completely Organic Multilayer Thin Film with Thermoelectric Power Factor Rivaling Inorganic Tellurides. Adv. Mater. 2015, 27, 2996–3001. [Google Scholar] [CrossRef]
- Paleo, A.J.; Vieira, E.M.F.; Wan, K.; Bondarchuk, O.; Cerqueira, M.F.; Goncalves, L.M.; Bilotti, E.; Alpuim, P.; Rocha, A.M. Negative thermoelectric power of melt mixed vapor grown carbon nanofiber polypropylene composites. Carbon 2019, 150, 408–416. [Google Scholar] [CrossRef]
- Paleo, A.J.; Vieira, E.M.F.; Wan, K.; Bondarchuk, O.; Cerqueira, M.F.; Bilotti, E.; Melle-Franco, M.; Rocha, A.M. Vapor grown carbon nanofiber based cotton fabrics with negative thermoelectric power. Cellulose 2020, 27, 9091–9104. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Adu, C.K.W.; Fang, S.; Eklund, P.C. Effects of Gas Adsorption and Collisions on Electrical Transport in Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2000, 85, 1096–1099. [Google Scholar] [CrossRef] [Green Version]
- Bradley, K.; Jhi, S.-H.; Collins, P.G.; Hone, J.; Cohen, M.L.; Louie, S.G.; Zettl, A. Is the Intrinsic Thermoelectric Power of Carbon Nanotubes Positive? Phys. Rev. Lett. 2000, 85, 4361–4364. [Google Scholar] [CrossRef] [Green Version]
- Krause, B.; Barbier, C.; Levente, J.; Klaus, M.; Pötschke, P. Screening of different carbon nanotubes in melt-mixed polymer composites with different polymer matrices for their thermoelectric properties. J. Compos. Sci. 2019, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- Brownlie, L.; Shapter, J. Advances in carbon nanotube n-type doping: Methods, analysis and applications. Carbon 2018, 126, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef]
- Paleo, A.J.; Krause, B.; Cerqueira, M.F.; Melle-Franco, M.; Pötschke, P.; Rocha, A.M. Thermoelectric properties of polypropylene carbon nanofiber melt-mixed composites: The effect of polymer on their Seebeck coefficient. Polym. J. 2021. submitted. [Google Scholar]
- Ayala, P.; Arenal, R.; Rümmeli, M.; Rubio, A.; Pichler, T. The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 2010, 48, 575–586. [Google Scholar] [CrossRef]
- Krause, B.; Konidakis, I.; Arjmand, M.; Sundararaj, U.; Fuge, R.; Liebscher, M.; Hampel, S.; Klaus, M.; Serpetzoglou, E.; Stratakis, E.; et al. Nitrogen-Doped Carbon Nanotube/Polypropylene Composites with Negative Seebeck Coefficient. J. Compos. Sci. 2020, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Tzounis, L.; Liebscher, M.; Fuge, R.; Leonhardt, A.; Mechtcherine, V. P- and n-type thermoelectric cement composites with CVD grown p- and n-doped carbon nanotubes: Demonstration of a structural thermoelectric generator. Energy Build. 2019, 191, 151–163. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, D.S.; Czerw, R.; Chiu, P.W.; Grobert, N.; Terrones, M.; Reyes-Reyes, M.; Terrones, H.; Charlier, J.C.; Ajayan, P.M.; et al. Nonlinear Behavior in the Thermopower of Doped Carbon Nanotubes Due to Strong, Localized States. Nano Lett. 2003, 3, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Piao, M.; Alam, M.R.; Kim, G.; Dettlaff-Weglikowska, U.; Roth, S. Effect of chemical treatment on the thermoelectric properties of single walled carbon nanotube networks. Phys. Status Solidi 2012, 249, 2353–2356. [Google Scholar] [CrossRef]
- Robeson, L.M. Applications of polymer blends: Emphasis on recent advances. Polym. Eng. Sci. 1984, 24, 587–597. [Google Scholar] [CrossRef]
- Ignacz, G.; Fei, F.; Szekely, G. Ion-Stabilized Membranes for Demanding Environments Fabricated from Polybenzimidazole and Its Blends with Polymers of Intrinsic Microporosity. ACS Appl. Nano Mater. 2018, 1, 6349–6356. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Y.C.; Wang, J.; Qi, H.J.; Wang, T.; Naguib, H.E. Flexible, Reconfigurable, and Self-Healing TPU/Vitrimer Polymer Blend with Copolymerization Triggered by Bond Exchange Reaction. ACS Appl. Mater. Interfaces 2020, 12, 8740–8750. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Y.; Wang, J.; Wang, C.; Liu, D.; Wang, G.; Liu, S. Mechanically Robust, Self-Healing, Polymer Blends and Polymer/Small Molecule Blend Materials with High Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 26966–26972. [Google Scholar] [CrossRef] [PubMed]
- Nyamweya, N.N. Applications of polymer blends in drug delivery. Future J. Pharm. Sci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Pionteck, J.; Pötschke, P.; Voit, B. Tuning the Structure and Performance of Bulk and Porous Vapor Sensors Based on Co-continuous Carbon Nanotube-Filled Blends of Poly(vinylidene fluoride) and Polycarbonates by Varying Melt Viscosity. ACS Appl. Mater. Interfaces 2020, 12, 45404–45419. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Hayakawa, Y.; Asai, S.; Miyasaka, K.; Tanemura, M. Double percolation effect on the electrical conductivity of conductive particles filled polymer blends. Colloid Polym. Sci. 1992, 270, 134–139. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S. Tuning the Conductivity of Nanocomposites through Nanoparticle Migration and Interface Crossing in Immiscible Polymer Blends: A Review on Fundamental Understanding. Macromol. Mater. Eng. 2019, 304, 1800431. [Google Scholar] [CrossRef]
- Göldel, A.; Marmur, A.; Kasaliwal, G.R.; Pötschke, P.; Heinrich, G. Shape-Dependent Localization of Carbon Nanotubes and Carbon Black in an Immiscible Polymer Blend during Melt Mixing. Macromolecules 2011, 44, 6094–6102. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Gumede, T.P.; Luyt, A.S.; Muller, A.J. Review on PCL, PBS, and PCL/PBS blends containing carbon nanotubes. Express Polym. Lett. 2018, 12, 505–529. [Google Scholar] [CrossRef]
- Bai, L.; He, S.; Fruehwirth, J.W.; Stein, A.; Macosko, C.W.; Cheng, X. Localizing graphene at the interface of cocontinuous polymer blends: Morphology, rheology, and conductivity of cocontinuous conductive polymer composites. J. Rheol. 2017, 61, 575–587. [Google Scholar] [CrossRef]
- Mamunya, Y.; Levchenko, V.; Boiteux, G.; Seytre, G.; Zanoaga, M.; Tanasa, F.; Lebedev, E. Controlling morphology, electrical, and mechanical properties of polymer blends by heterogeneous distribution of carbon nanotubes. Polym. Compos. 2016, 37, 2467–2477. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, B.-Y.; Xiong, Z.-Y.; Guo, Z.-X.; Yu, J. Preparation of conductive polyphenylene sulfide/polyamide 6/multiwalled carbon nanotube composites using the slow migration rate of multiwalled carbon nanotubes from polyphenylene sulfide to polyamide 6. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.R.; Pötschke, P.; Heinrich, G. The kinetics of CNT transfer between immiscible blend phases during melt mixing. Polymer 2012, 53, 411–421. [Google Scholar] [CrossRef]
- Yu, C.; Kim, Y.S.; Kim, D.; Grunlan, J.C. Thermoelectric Behavior of Segregated-Network Polymer Nanocomposites. Nano Lett. 2008, 8, 4428–4432. [Google Scholar] [CrossRef]
- Pang, H.; Piao, Y.-Y.; Tan, Y.-Q.; Jiang, G.-Y.; Wang, J.-H.; Li, Z.-M. Thermoelectric behaviour of segregated conductive polymer composites with hybrid fillers of carbon nanotube and bismuth telluride. Mater. Lett. 2013, 107, 150–153. [Google Scholar] [CrossRef]
- Krause, B.; Pötschke, P.; Ilin, E.; Predtechenskiy, M. Melt mixed SWCNT-polypropylene composites with very low electrical percolation. Polymer 2016, 98, 45–50. [Google Scholar] [CrossRef]
- Taylor, G.I. The viscosity of a fluid containing small drops of another fluid. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1932, 138, 41–48. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion Phenomena in High Viscosity Immiscible Fluid Systems and Application of Static Mixers as Dispersion Devices in such Systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Arrigo, R.; Malucelli, G. Rheological Behavior of Polymer/Carbon Nanotube Composites: An Overview. Materials 2020, 13, 2771. [Google Scholar] [CrossRef] [PubMed]
- Valentino, O.; Sarno, M.; Rainone, N.G.; Nobile, M.R.; Ciambelli, P.; Neitzert, H.C.; Simon, G.P. Influence of the polymer structure and nanotube concentration on the conductivity and rheological properties of polyethylene/CNT composites. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 2440–2445. [Google Scholar] [CrossRef]
- Krause, B.; Barbier, C.; Kunz, K.; Pötschke, P. Comparative study of singlewalled, multiwalled, and branched carbon nanotubes melt mixed in different thermoplastic matrices. Polymer 2018, 159, 75–85. [Google Scholar] [CrossRef]
- Kunz, K.; Krause, B.; Kretzschmar, B.; Juhasz, L.; Kobsch, O.; Jenschke, W.; Ullrich, M.; Pötschke, P. Direction Dependent Electrical Conductivity of Polymer/Carbon Filler Composites. Polymers 2019, 11, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjmand, M.; Chizari, K.; Krause, B.; Pötschke, P.; Sundararaj, U. Effect of synthesis catalyst on structure of nitrogen-doped carbon nanotubes and electrical conductivity and electromagnetic interference shielding of their polymeric nanocomposites. Carbon 2016, 98, 358–372. [Google Scholar] [CrossRef]
- Arjmand, M.; Sundararaj, U. Electromagnetic interference shielding of Nitrogen-doped and Undoped carbon nanotube/polyvinylidene fluoride nanocomposites: A comparative study. Compos. Sci. Technol. 2015, 118, 257–263. [Google Scholar] [CrossRef]
- Göldel, A.; Kasaliwal, G.; Pötschke, P. Selective Localization and Migration of Multiwalled Carbon Nanotubes in Blends of Polycarbonate and Poly(styrene-acrylonitrile). Macromol. Rapid Commun. 2009, 30, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, M.; Tzounis, L.; Pötschke, P.; Heinrich, G. Influence of the viscosity ratio in PC/SAN blends filled with MWCNTs on the morphological, electrical, and melt rheological properties. Polymer 2013, 54, 6801–6808. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.; Bondre, A.; Kulkarni, A.; Pötschke, P. Rheology, electrical conductivity, and the phase behavior of cocontinuous PA6/ABS blends with MWNT: Correlating the aspect ratio of MWNT with the percolation threshold. J. Polym. Sci. Part. B Polym. Phys. 2008, 46, 1619–1631. [Google Scholar] [CrossRef]
- Monemian, S.; Jafari, S.H.; Khonakdar, H.; Gooadrzi, V.; Reuter, U.; Pötschke, P. MWNT-filled PC/ABS blends: Correlation of morphology with rheological and electrical response. J. Appl. Polym. Sci. 2013, 130. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker Inc.: New York, NY, USA, 1982. [Google Scholar]
- Nuriel, S.; Liu, L.; Barber, A.H.; Wagner, H.D. Direct measurement of multiwall nanotube surface tension. Chem. Phys. Lett. 2005, 404, 263–266. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Static and Dynamic Wetting Measurements of Single Carbon Nanotubes. Phys. Rev. Lett. 2004, 92, 186103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudouin, A.-C.; Devaux, J.; Bailly, C. Localization of carbon nanotubes at the interface in blends of polyamide and ethylene-acrylate copolymer. Polymer 2010, 51, 1341–1354. [Google Scholar] [CrossRef]
- Mehrabi Mazidi, M.; Edalat, A.; Berahman, R.; Hosseini, F.S. Highly-Toughened Polylactide- (PLA-) Based Ternary Blends with Significantly Enhanced Glass Transition and Melt Strength: Tailoring the Interfacial Interactions, Phase Morphology, and Performance. Macromolecules 2018, 51, 4298–4314. [Google Scholar] [CrossRef]
- Pionteck, J.; Kreßler, J. Interfacial Tension and Miscibility of Polymer Blends. In Proceedings of the EPS’97—European Physical Society “Surfaces and Interfaces in Polymers and Composites”, Lausanne, Switzerland, 1–6 June 1997; pp. 31–32. [Google Scholar]
- Wu, S. Surface and interfacial tensions of polymer melts. II. Poly(methyl methacrylate), poly(n-butyl methacrylate), and polystyrene. J. Phys. Chem. 1970, 74, 632–638. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Hybart, F.J.; White, T.R. The surface tension of viscous polymers at high temperatures. J. Appl. Polym. Sci. 1960, 3, 118–121. [Google Scholar] [CrossRef]
- Son, Y. Measurement of interfacial tension between polyamide-6 and poly(styrene-co-acrylonitrile) by breaking thread method. Polymer 2001, 42, 1287–1291. [Google Scholar] [CrossRef]
- Wu, S. Interfacial energy, structure, and adhesion between polymers (chapter 6). In Polymer Blends; Paul, D.R., Newman, S., Eds.; Academic Press, Inc.: New York, NY, USA, 1978; Volume 1, pp. 244–295. [Google Scholar]












| Blend Ratio | Set 1 SWCNT Content in Whole Blend [wt.%] | Set 1 SWCNT Content in PA6 [wt.%] | Set 2 SWCNT Content in Whole Blend [wt.%] | Set 2 SWCNT Content in PA6 [wt.%] |
|---|---|---|---|---|
| 90/10 | 3 | 3.33 | 2.7 | 3 |
| 80/20 | 3 | 3.75 | 2.4 | 3 |
| 70/30 | 3 | 4.29 | 2.1 | 3 |
| 60/40 | 3 | 5.0 | 1.8 | 3 |
| 50/50 | 3 | 6.0 | 1.5 | 3 |
| 50/50 | 1 | 2 | ||
| 50/50 | 0.5 | 1 | ||
| 50/50 | 0.25 | 0.5 |
| Blend Ratio | PA6/SAN +3 wt.% | PA6/SAN +<3 wt.% | PA6/PMMA +3 wt.% | PA6/PMMA +<3 wt.% |
|---|---|---|---|---|
| 100/0 | 2.5 ± 1.9 | 2.5 ± 1.9 | ||
| 90/10 | 2.6 ± 1.8 | 0.8 ± 0.4 | 1.7 ± 0.5 | 1.0 ± 0.6 |
| 80/20 | 2.2 ± 1.2 | 0.5 ± 0.2 | 2.8 ± 1.0 | 1.4 ± 0.6 |
| 70/30 | 1.9 ± 0.4 | 1.5 ± 1.1 | 2.0 ± 0.5 | 1.0 ± 0.7 |
| 60/40 | 2.0 ± 0.8 | 1.3 ± 0.6 | 3.2 ± 1.1 | 1.3 ± 0.6 |
| 50/50 | 3.5 ± 1.1 | 1.5 ± 0.5 | 2.1 ± 0.5 | 1.7 ± 0.9 |
| Material 1 | Surface Tension Total γ [mN/m] | Surface Tension Disperse Part γd [mN/m] | Surface Tension Polar Part γP [mN/m] |
|---|---|---|---|
| PA6 | 35.1 | 25.3 | 9.9 |
| SAN 1 | 25.3 | 20.6 | 4.8 |
| SAN 2 | 28.5 | 22.8 | 5.7 |
| PMMA 1 | 23.2 | 18.9 | 4.4 |
| PMMA 2 | 21.3 | 15.3 | 6.0 |
| MWCNT 1 | 45.3 | 18.4 | 26.9 |
| MWCNT 2 | 27.8 | 17.6 | 10.2 |
| Materials | Interfacial Tension γ12, Geometric Mean Value [mN/m] | Interfacial Tension γ12, Harmonic Mean Value [mN/m] |
|---|---|---|
| MWCNT 1-PA6 | 4.72 | 8.96 |
| MWCNT 2-PA6 | 0.69 | 1.37 |
| MWCNT 1-SAN 1 | 9.10 | 15.62 |
| MWCNT 2-SAN 1 | 1.14 | 2.22 |
| MWCNT 1-SAN 2 | 8.07 | 14.26 |
| MWCNT 2-SAN 2 | 0.99 | 1.94 |
| MWCNT 1-PMMA 1 | 9.19 | 15.71 |
| MWCNT 2-PMMA 1 | 1.09 | 2.11 |
| MWCNT 1-PMMA 1 | 7.66 | 13.59 |
| MWCNT 2-PMMA 1 | 0.64 | 1.26 |
| PA6-SAN 1 | 1.17 | 2.27 |
| PA6-SAN 2 | 0.63 | 1.24 |
| PA6-PMMA 1 | 1.49 | 2.90 |
| PA6-PMMA 2 | 1.71 | 3.37 |
| Composition | Wetting Coefficient ωa, Geometric Mean Value [−] | Wetting Coefficient ωa, Harmonic Mean Value [−] |
|---|---|---|
| PA6/SAN 1/MWCNT 1 | 3.76 | 2.93 |
| PA6/SAN 1/MWCNT 2 | 0.39 | 0.37 |
| PA6/SAN 2/MWCNT 1 | 5.3 | 4.26 |
| PA6/SAN 2/MWCNT 2 | 0.47 | 0.46 |
| PA6/PMMA 1/MWCNT 1 | 3.01 | 2.32 |
| PA6/PMMA 1/MWCNT 2 | 0.27 | 0.25 |
| PA6/PMMA 2/MWCNT 1 | 1.72 | 1.38 |
| PA6/PMMA 2/MWCNT 2 | −0.03 | −0.03 |
| Sample | Density [g/cm3] | Temperature Conductivity [mm2/s] | Specific Heat Capacity [J/g·K] | Thermal Conductivity [W/m·K] |
|---|---|---|---|---|
| PA6 | 1.12 | 0.17 ± 0.03 | 1.62 ± 0.08 | 0.31 ± 0.04 |
| SAN | 1.05 | 0.13 ± 0.00 | 1.50 ± 0.01 | 0.20 ± 0.00 |
| PMMA | 1.11 | 0.14 ± 0.00 | 1.58 ± 0.00 | 0.25 ± 0.00 |
| PA6/SAN 50/50 | 1.05 | 0.16 ± 0.01 | 1.65 ± 0.03 | 0.27 ± 0.02 |
| PA6/PMMA 50/50 | 1.12 | 0.16 ± 0.00 | 1.50 ± 0.00 | 0.27 ± 0.00 |
| PA6 +3 wt.% SWCNT | 1.16 | 0.36 ± 0.01 | 1.48 ± 0.01 | 0.62 ± 0.03 |
| PA6/SAN 50/50 +3 wt.% SWCNT | 1.11 | 0.27 ± 0.01 | 1.47 ± 0.05 | 0.45 ± 0.01 |
| PA6/PMMA 50/50 +3 wt.% SWCNT | 1.17 | 0.28 ± 0.01 | 1.45 ± 0.02 | 0.48 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause, B.; Liguoro, A.; Pötschke, P. Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes. Nanomaterials 2021, 11, 1146. https://doi.org/10.3390/nano11051146
Krause B, Liguoro A, Pötschke P. Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes. Nanomaterials. 2021; 11(5):1146. https://doi.org/10.3390/nano11051146
Chicago/Turabian StyleKrause, Beate, Alice Liguoro, and Petra Pötschke. 2021. "Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes" Nanomaterials 11, no. 5: 1146. https://doi.org/10.3390/nano11051146
APA StyleKrause, B., Liguoro, A., & Pötschke, P. (2021). Blend Structure and n-Type Thermoelectric Performance of PA6/SAN and PA6/PMMA Blends Filled with Singlewalled Carbon Nanotubes. Nanomaterials, 11(5), 1146. https://doi.org/10.3390/nano11051146