Modified Camellia oleifera Shell Carbon with Enhanced Performance for the Adsorption of Cooking Fumes
Abstract
:1. Introduction
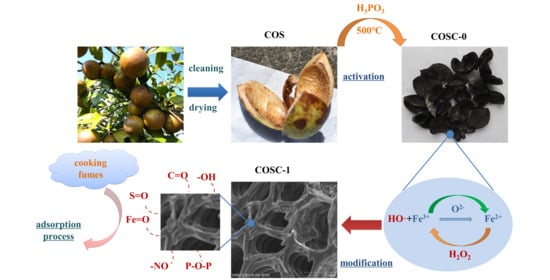
1.1. Preparation and Modification of COSC
1.2. Concentration Measurement of Pollutants from Cooking Fumes
1.3. Measurement of Adsorption Capacity
1.4. GC-MS Analysis of Organic Contents in Cooking Fumes
1.5. Characterization
2. Results and Analysis
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, S.; Wu, J.; Liu, G.; Zhang, B.; Zheng, M. Unintentionally produced dioxin-like polychlorinated biphenyls during cooking. Food Control 2011, 22, 1797–1802. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, S.; Wu, Y.; Huai, H. The effects of cooking oil fume condensates (COFCs) on the vegetative growth of Salvinia natans (L.) All. J. Hazard. Mater. 2009, 172, 240–246. [Google Scholar] [CrossRef]
- Katragadda, H.R.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, Á.A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, Z.; Guan, P.; Li, X.; Wu, W.; Ren, Y.; Zhou, B. Interaction between polymorphisms in pre-MiRNA genes and cooking oil fume exposure on the risk of lung cancer in Chinese non-smoking female population. PLoS ONE 2015, 10, e0128572. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yin, Z.; Li, K.; Wan, Y.; Li, X.; Wu, W.; Guan, P.; Zhou, B. TGFβ-1 and TGFBR2 polymorphisms, cooking oil fume exposure and risk of lung adenocarcinoma in Chinese nonsmoking females: A case control study. BMC Med. Genet. 2015, 16, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couraud, S.; Zalcman, G.; Milleron, B.; Morin, F.; Souquet, P.-J. Lung cancer in never smokers-A review. Eur. J. Cancer 2012, 48, 1299–1311. [Google Scholar] [CrossRef]
- Xue, X.; Yin, Z.; Lü, Y.; Zhang, H.; Yan, Y.; Zhao, Y.; Li, X.; Cui, Z.; Yu, M.; Yao, L.; et al. The Joint Effect of hOGG1, APE1, and ADPRT Polymorphisms and Cooking Oil Fumes on the Risk of Lung Adenocarcinoma in Chinese Non-Smoking Females. PLoS ONE 2013, 8, e71157. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Hsieh, C.-C. Integration of chemical scrubber with sodium hypochlorite and surfactant for removal of hydrocarbons in cooking oil fume. J. Hazard. Mater. 2010, 182, 39–44. [Google Scholar] [CrossRef]
- Wang, J.; Liao, C.; Chen, Y.; Cao, H.; Liu, Z.; Gong, M.; Chen, Y. Low-temperature catalytic combustion of cooking fume over Pt/gamma-Al2O3/Ce0.5-xZr0.5-xMn2xO2 monolithic catalyst. Chin. J. Catal. 2010, 31, 404–408. [Google Scholar]
- Wu, M.-T.; Lee, L.-H.; Ho, C.-K.; Wu, S.-C.; Lin, L.-Y.; Cheng, B.-H.; Liu, C.-L.; Yang, C.-Y.; Tsai, H.-T.; Wu, T.-N. Environmental exposure to cooking oil fumes and cervical intraepithelial neoplasm. Environ. Res. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Boehm, H. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Suzuki, R.; Andrade, A.; Sousa, J.; Rollemberg, M. Preparation and characterization of activated carbon from rice bran. Bioresour. Technol. 2007, 98, 1985–1991. [Google Scholar] [CrossRef]
- Oh, G.H.; Park, C.R. Preparation and characteristics of rice-straw-based porous carbons with high adsorption capacity. Fuel 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.; Rao, R. Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties1Louisiana Agricultural Experiment Station manuscript 99-21-0066.1. Bioresour. Technol. 2000, 71, 113–123. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D. The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon 1997, 35, 1723–1732. [Google Scholar] [CrossRef]
- Ahmedna, M.; E Marshall, W.; Husseiny, A.A.; Rao, R.M.; Goktepe, I. The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res. 2004, 38, 1062–1068. [Google Scholar] [CrossRef]
- Savova, D.; Apak, E.; Ekinci, E.; Yardim, F.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass conversion to carbon adsorbents and gas. Biomass Bioenergy 2001, 21, 133–142. [Google Scholar] [CrossRef]
- Marcilla, A.; García-García, S.; Asensio, M.; Conesa, J. Influence of thermal treatment regime on the density and reactivity of activated carbons from almond shells. Carbon 2000, 38, 429–440. [Google Scholar] [CrossRef]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Minkova, V.; Razvigorova, M.; Bjornbom, E.; Zanzi, R.; Budinova, T.; Petrov, N. Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Process. Technol. 2001, 70, 53–61. [Google Scholar] [CrossRef]
- Minkova, V.; Marinov, S.; Zanzi, R.; Björnbom, E.; Budinova, T.; Stefanova, M.; Lakov, L. Thermochemical treatment of biomass in a flow of steam or in a mixture of steam and carbon dioxide. Fuel Process. Technol. 2000, 62, 45–52. [Google Scholar] [CrossRef]
- Jin, X. Bioactivities of water-soluble polysaccharides from fruit shell of Camellia oleifera Abel: Antitumor and antioxidant activities. Carbohydr. Polym. 2012, 87, 2198–2201. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J.C.; Cui, D.D. Preparation of activated carbon with highly developed mesoporous structure from Camellia oleifera shell through water vapor gasification and phosphoric acid modification. Biomass Bioenergy 2011, 35, 3643–3647. [Google Scholar]
- Zheng, Z.; Zhao, H.; Lin, X.; Yang, J.; Shi, R. Preparation of activated carbon from Camellia oleifera shell and its application to adsorption of hexavalent chromium from aqueous solution: Kinetics, equilibrium, and thermodynamics. Desalin Water Treat. 2020, 198, 170–179. [Google Scholar] [CrossRef]
- Mei, L.; Qiao, H.; Ke, F.; Peng, C.; Hou, R.; Wan, X.; Cai, H. One-step synthesis of zirconium dioxide-biochar derived from Camellia oleifera seed shell with enhanced removal capacity for fluoride from water. Appl. Surf. Sci. 2020, 509, 144685. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Cui, T.; He, W.-Q.; Nie, L.; Wang, J.-L.; Pan, T. Pollution Characteristics of Aldehydes and Ketones Compounds in the Exhaust of Beijing Typical Restaurants. Huanjing Kexue 2015, 36, 2743–2749. [Google Scholar]
- Zheng, S.; Luo, Y.; Zhang, K.; Liu, H.; Hu, G.; Qin, A. Nitrogen and phosphorus co-doped mesoporous carbon nanosheets derived from bagasse for lithium-ion batteries. Mater. Lett. 2021, 290, 129459. [Google Scholar] [CrossRef]
- Fan, Y.H.; Yu, N.H.; Deng, L.Y. Preparation of High Mesoporous Activated Carbon from Camellia oleifera Shell. J. Northwest For. Univ. 2019, 34, 187–194. [Google Scholar]
- Morenocastilla, C.; Ferrogarcia, M.A.; Joly, J.P.; Bautistatoledo, I.; Carrascomarin, F.; Riverautrilla, J. Activated Carbon Surface Modifications by Nitric Acid, Hydrogen Peroxide, and Ammonium Peroxydisulfate Treatments. Langmuir 1995, 11, 4386–4392. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Jagiełło, J.; Schwarz, J.A.; Krzyzanowski, A. Effect of Surface Chemistry on Sorption of Water and Methanol on Activated Carbons. Langmuir 1996, 12, 6480–6486. [Google Scholar] [CrossRef]
- Yao, R.P.; Zhang, M.J.; Yang, J.; Yi, D.L.; Xu, J.; Deng, F.; Yue, Y.; Ye, C.H. Preparation of SO3/gamma-Al2O3 solid acid catalyst and characterization of its structure and acidity. Acta Chim. Sin. 2005, 64, 269–273. [Google Scholar]
- Krehula, S.; Music, S. Influence of ruthenium ions on the precipitation of alpha-FeOOH, alpha-Fe2O3 and Fe3O4 in highly alkaline media. J. Alloy. Compd. 2006, 416, 284–290. [Google Scholar] [CrossRef]
- Sudakar, C.; Subbanna, G.N.; Kutty, T.R.N. Synthesis of acicular hydrogoethite (α-FeOOH·xH2O; 0.1 < x < 0.22) particles using morphology controlling cationic additives and magnetic properties of maghemite derived from hydrogoethite. J. Mater. Chem. 2001, 12, 107–116. [Google Scholar]
- You, J.H.; Chiang, H.L.; Chiang, P.C. Comparison of adsorption characteristics for VOCs on activated carbon and oxidized activated carbon. Environ. Prog. 1994, 13, 31–36. [Google Scholar] [CrossRef]
- Pan, B.; Xing, B. Adsorption Mechanisms of Organic Chemicals on Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef]
- Cong, Y.; Wu, Z. Electrocatalytic Generation of Radical Intermediates over Lead Dioxide Electrode Doped with Fluoride. J. Phys. Chem. C 2007, 111, 3442–3446. [Google Scholar] [CrossRef]
- Ma, N.; Tian, Y.-J.; Yang, G.-P.; Xie, X.-Y. Modification of activated carbon fiber for electro-Fenton degradation of phenol. Huanjing kexue 2014, 35, 2627–2632. [Google Scholar]
- Trinh, Q.H.; Lee, S.B.; Mok, Y.S. Removal of ethylene from air stream by adsorption and plasma-catalytic oxidation using silver-based bimetallic catalysts supported on zeolite. J. Hazard. Mater. 2015, 285, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, P.; Xu, W.; Wu, J.; Chen, L.; Fu, M.; Ye, D. Plasma-catalysis of metal loaded SBA-15 for toluene removal: Comparison of continuously introduced and adsorption-discharge plasma system. Chem. Eng. J. 2016, 283, 276–284. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, Y.; Tang, X.; Yi, H.; Zhao, S.; Gao, F.; Zhou, Y.; Zhang, Y.; Zhuang, R. A novel ferrisilicate MEL zeolite with bi-functional adsorption/catalytic oxidation properties for non-methane hydrocarbon removal from cooking oil fumes. Microporous Mesoporous Mater. 2020, 309, 110509. [Google Scholar] [CrossRef]








| No. | t (min) | Name of Chemical Compound | Molecular Formula | Removal Rate (%) | ||
|---|---|---|---|---|---|---|
| COS | COSC-0 | COSC-1 | ||||
| 1 | 3.189 | 2, 7-dimethyl-1-octanol | C10H22O | 100 | 100 | 100 |
| 2 | 4.965 | n-heptaldehyde | C7H14O | 100 | 100 | 100 |
| 3 | 6.263 | (2Z)-Heptenal | C7H12O | 100 | 100 | 100 |
| 4 | 7.051 | 2-Pentylfuran | C9H14O | 40 | 67 | 100 |
| 5 | 7.421 | Octanal | C8H16O | 100 | 100 | 100 |
| 6 | 8.949 | (E)-2-Octena | C8H14O | 100 | 100 | 100 |
| 7 | 10.7 | 1-hexyl-1-cyclopentene | C11H20 | 67 | 48 | 100 |
| 8 | 11.58 | p-propenyl phenyl methyl ether | C10H12O | 27 | 37 | 46 |
| 9 | 11.8 | trans-2-nonenal | C9H16O | 100 | 100 | 100 |
| 10 | 14.58 | trans-2-decyl olefine aldehyde | C10H18O | 100 | 100 | 100 |
| 11 | 15.35 | 2-octyl-tetrahydro-furan | C12H20O | 100 | 100 | 100 |
| 12 | 16.11 | 2, 4-decadienal | C10H16O | 100 | 100 | 100 |
| 13 | 17.27 | 2-undecenal | C11H20O | 100 | 100 | 100 |
| 14 | 20.78 | N-benzal-allyl amine | C10H11N | 31 | 46 | 53 |
| 15 | 26.92 | 1, 2-diphenyl cyclopropane | C15H14 | 22 | 31 | 43 |
| 16 | 27.93 | linalyl isobutyrate | C14H24O2 | 100 | 100 | 100 |
| 17 | 28.72 | 3-DNA-estradiol | C18H24O | 27 | 28 | 44 |
| 18 | 29.08 | 1, 5-diphenyl-3-(2-ethyl benzene)-2-amylene | C25H26 | 30 | 40 | 50 |
| 19 | 29.42 | timnodonic acid | C20H30O2 | 22 | 33 | 45 |
| 20 | 30.41 | 5, 7-dodecane acetylene 2-1, 12-dio | C12H18O2 | 29 | 36 | 52 |
| 21 | 31.02 | [(2, 3-diphenyl propyl) methyl]-phenyl sulfur | C22H20OS | 26 | 100 | 100 |
| 22 | 33.34 | 2-methyl-6-benzene-1, 6-heptyl diene | C14H18 | 41 | 52 | 64 |
| Samples | COSC-0 | COSC-1 |
|---|---|---|
| SBET/(m2/g) | 1245 | 934 |
| Vtotal/(cm3/g) | 1.02 | 0.69 |
| Dp/(nm) | 3.284 | 4.021 |
| Adsorbent | SBET/(m2/g) | Vtotal/(cm3/g) | PAQ/(mg/g) | References |
|---|---|---|---|---|
| COSC-0 | 1244.7 | 1.02 | 6.43 | This study |
| COSC-1 | 933.5 | 0.69 | 22.58 | This study |
| Sam-SiFe (II) | 388.0 | 0.23 | 4.468 | [43] |
| Sam-SiFe (III) | 411.4 | 0.23 | 3.659 | [43] |
| Sam-SiAl | 403.6 | 0.23 | 2.781 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, D.; Shi, W.; Gao, J.; Deng, B.; Yu, R. Modified Camellia oleifera Shell Carbon with Enhanced Performance for the Adsorption of Cooking Fumes. Nanomaterials 2021, 11, 1349. https://doi.org/10.3390/nano11051349
Liao D, Shi W, Gao J, Deng B, Yu R. Modified Camellia oleifera Shell Carbon with Enhanced Performance for the Adsorption of Cooking Fumes. Nanomaterials. 2021; 11(5):1349. https://doi.org/10.3390/nano11051349
Chicago/Turabian StyleLiao, Dongliang, Wen Shi, Jing Gao, Bin Deng, and Ruijin Yu. 2021. "Modified Camellia oleifera Shell Carbon with Enhanced Performance for the Adsorption of Cooking Fumes" Nanomaterials 11, no. 5: 1349. https://doi.org/10.3390/nano11051349
APA StyleLiao, D., Shi, W., Gao, J., Deng, B., & Yu, R. (2021). Modified Camellia oleifera Shell Carbon with Enhanced Performance for the Adsorption of Cooking Fumes. Nanomaterials, 11(5), 1349. https://doi.org/10.3390/nano11051349