pH-Responsive Nanoemulsions Based on a Dynamic Covalent Surfactant
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of T−DBA
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy Measurement
2.4. 1H NMR Measurement
2.5. Surface Tension Measurement
2.6. Dynamic Interfacial Tension (IFT) Measurement
2.7. Nanoemulsion Preparation
2.8. Dynamic Light Scattering (DLS) Measurement
2.9. Zeta Potential Measurement
2.10. pH Adjustment Method
3. Results and Discussion
3.1. Preparation and Characterization of T−DBA
3.2. The Critical Micelle Concentration (CMC) of T−DBA
3.3. The Emulsification Ability of the T−DBA Surfactant
3.4. pH-Responsive Nanoemulsions
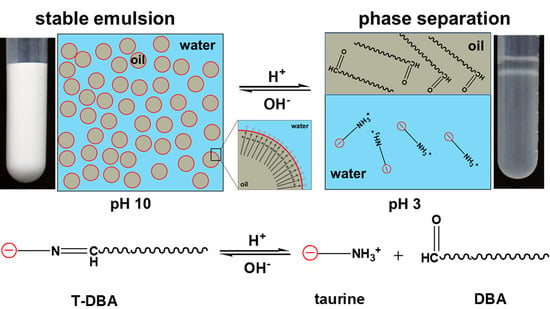
3.5. Mechanism of pH-Responsive Nanoemulsions
3.6. Universality of This Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Onaizi, S.A. Demulsification of crude oil/water nanoemulsions stabilized by rhamnolipid biosurfactant using enzymes and pH-swing. Sep. Purif. Technol. 2021, 259, 118060. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Oil-in-water nanoemulsion stabilized by polymeric surfactant: Characterization and properties evaluation for enhanced oil recovery. Eur. Polym. J. 2018, 109, 265–276. [Google Scholar] [CrossRef]
- Liang, J.; Du, N.; Song, S.; Hou, W. Magnetic demulsification of diluted crude oil-in-water nanoemulsions using oleic acid-coated magnetite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 197–202. [Google Scholar] [CrossRef]
- Liu, Y. Switchable Surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Alsulaimani, M.; Al-Sakkaf, M.K.; Bahadi, S.A.; Mahmoud, M.; Alshami, A. Crude oil/water nanoemulsions stabilized by biosurfactant: Stability and pH-Switchability. J. Pet. Sci. Eng. 2021, 198, 108173. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Z.; Xu, Z.; Hao, J.; Sun, D. Highly effective emulsification/demulsification with a CO2-switchable superamphiphile. J. Colloid Interface Sci. 2016, 480, 198–204. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Jiang, J.; Cui, Z.; Xia, W. Redox-Responsive Oil-In-Dispersion Emulsions Stabilized by Similarly Charged Ferrocene Surfactants and Alumina Nanoparticles. Langmuir 2020, 36, 14589–14596. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A.; Hejazi, S.H.; Gates, I. Dual Stimuli-Responsive Pickering Emulsions from Novel Magnetic Hydroxyapatite Nanoparticles and Their Characterization Using a Microfluidic Platform. Langmuir 2021, 37, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Liu, B.; Jiang, H. Sodium caseinate as a particulate emulsifier for making indefinitely recycled pH-responsive emulsions. Chem. Sci. 2020, 11, 3797–3803. [Google Scholar] [CrossRef]
- Geng, J.; Pu, J.; Zhao, Y.; Lin, B.; Bai, B.; Thomas, S.P. pH-Responsive crude oil-in-water Pickering emulsion stabilized by polyacrylamide nanogels. Fuel 2019, 258, 116159. [Google Scholar] [CrossRef]
- Ren, G.; Wang, M.; Wang, L.; Wang, Z.; Chen, Q.; Xu, Z.; Sun, D. Dynamic Covalent Silica Nanoparticles for pH-Switchable Pickering Emulsions. Langmuir 2018, 34, 5798–5806. [Google Scholar] [CrossRef]
- Ren, G.; Wang, L.; Chen, Q.; Xu, Z.; Xu, J.; Sun, D. pH Switchable Emulsions Based on Dynamic Covalent Surfactants. Langmuir 2017, 33, 3040–3046. [Google Scholar] [CrossRef]
- Richtering, W. Responsive Emulsions Stabilized by Stimuli-Sensitive Microgels: Emulsions with Special Non-Pickering Properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef]
- Kwok, M.; Ngai, T. Emulsions stabilized by pH-responsive PNIPAM-based microgels: Effect of spatial distribution of functional carboxylic groups on the emulsion stability. J. Taiwan Inst. Chem. Eng. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Manica, R.; Liu, Q.; Xu, Z. Enhancing oil-solid and oil-water separation in heavy oil recovery by CO2-responsive surfactants. AIChE J. 2021, 67, e17033. [Google Scholar] [CrossRef]
- Brugger, B.; Richtering, W. Magnetic, Thermosensitive Microgels as Stimuli-Responsive Emulsifiers Allowing for Remote Control of Separability and Stability of Oil in Water-Emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, X.; Yang, S.; Zhang, S.; Yu, G.; Liu, Y.; Zhou, Y.; Feng, Y.; Li, J. Light-tuning amphiphility of host-guest Alginate-based supramolecular assemblies for photo-responsive Pickering emulsions. Carbohydr. Polym. 2021, 251, 117072. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhu, A.; Zhao, Y.; Wang, H.; Binks, B.P.; Wang, J. Light-Responsive, Reversible Emulsification and Demulsification of Oil-in-Water Pickering Emulsions for Catalysis. Angew. Chem. Int. Ed. 2021, 60, 3928–3933. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Ren, X.; Liu, X.; Fang, Y. CO2 and Redox Dual Responsive Pickering Emulsion. Langmuir 2017, 33, 12973–12981. [Google Scholar] [CrossRef]
- Tan, B.H.; Tam, K.C. Review on the dynamics and micro-structure of pH-responsive nano-colloidal systems. Adv. Colloid Interface Sci. 2008, 136, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W.; Yang, H. Effects of surface modification Nano-SiO2 and its combination with surfactant on interfacial tension and emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124682. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K.; Lu, Y.; Meng, Z.; Gou, C.; Li, Z.; Yang, M.; Qu, M.; Liu, T.; Hou, J.; et al. Silica-based amphiphilic Janus nanofluid with improved interfacial properties for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124162. [Google Scholar] [CrossRef]
- Pal, N.; Mandal, A. Enhanced oil recovery performance of gemini surfactant-stabilized nanoemulsions functionalized with partially hydrolyzed polymer/silica nanoparticles. Chem. Eng. Sci. 2020, 226, 115887. [Google Scholar] [CrossRef]
- Sun, X.; Li, S.; Liu, Z.; Wang, P.; Pei, S.; Zhang, J. Design of pH-responsive “on-off” emulsions using CTAB/PPA emulsifiers by simulations and experiments. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 140–146. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, D.; Dai, S.; Wang, B.; Lu, H.; Huang, Z. Redox and pH Dual-Responsive Emulsion Using Ferrocenecarboxylic Acid and N,N-Dimethyldodecylamine. Langmuir 2020, 36, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Harjani, J.R.; Liang, C.; Jessop, P.G. A Synthesis of Acetamidines. J. Org. Chem. 2011, 76, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Zentner, C.A.; Anson, F.; Thayumanavan, S.; Swager, T.M. Dynamic Imine Chemistry at Complex Double Emulsion Interfaces. J. Am. Chem. Soc. 2019, 141, 18048–18055. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, R.; Hu, J.; Wei, Z.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. One-Step Dynamic Imine Chemistry for Preparation of Chitosan-Stabilized Emulsions Using a Natural Aldehyde: Acid Trigger Mechanism and Regulation and Gastric Delivery. J. Agric. Food Chem. 2020, 68, 5412–5425. [Google Scholar] [CrossRef]
- Lu, P.; He, S.; Zhou, Y.; Zhang, Y. Oxidation-Induced Breakage of the Imine Bond and Aggregate Transition in a Se-Containing Dynamic Covalent Surfactant. Langmuir 2021, 37, 2833–2842. [Google Scholar] [CrossRef]
- Minkenberg, C.B.; Li, F.; van Rijn, P.; Florusse, L.; Boekhoven, J.; Stuart, M.C.A.; Koper, G.J.M.; Eelkema, R.; van Esch, J.H. Responsive Vesicles from Dynamic Covalent Surfactants. Angew. Chem. Int. Ed. 2011, 50, 3421–3424. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, N.; Saw, R.K.; Mandal, A. Gemini surfactant/polymer/silica stabilized oil-in-water nanoemulsions: Design and physicochemical characterization for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 183, 106464. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Yu, L.; Li, C.; Xu, J.; Hao, J.; Sun, D. Highly Stable Concentrated Nanoemulsions by the Phase Inversion Composition Method at Elevated Temperature. Langmuir 2012, 28, 14547–14552. [Google Scholar] [CrossRef]
- Espinosa-Sandoval, L.; Ochoa-Martínez, C.; Ayala-Aponte, A.; Pastrana, L.; Gonçalves, C.; Cerqueira, M.A. Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment. Nanomaterials 2021, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Cheraghian, G. Improved heavy oil recovery by nanofluid surfactant flooding-an experimental study. In Proceedings of the 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016; European Association of Geoscientists & Engineers Austria: Houten, The Netherlands, 2016; Volume 2016, pp. 1–5. [Google Scholar]
- Zhou, L.; Chen, M.; Guan, Y.; Zhang, Y. Multiple responsive hydrogel films based on dynamic Schiff base linkages. Polym. Chem. 2014, 5, 7081–7089. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Wang, Z.; Zhang, X. A pH-Responsive Superamphiphile Based on Dynamic Covalent Bonds. Chem. A Eur. J. 2011, 17, 3322–3325. [Google Scholar] [CrossRef]
- Acharyya, K.; Mukherjee, S.; Mukherjee, P.S. Molecular Marriage through Partner Preferences in Covalent Cage Formation and Cage-to-Cage Transformation. J. Am. Chem. Soc. 2012, 135, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y. A Facile Route towards the Preparation of Ultra-Long-Chain Amidosulfobetaine Surfactants. Synlett 2009, 2009, 2655–2658. [Google Scholar]
- Daneshvar, N.; Shirini, F.; Langarudi, M.S.N.; Karimi-Chayjani, R. Taurine as a green bio-organic catalyst for the preparation of bio-active barbituric and thiobarbituric acid derivatives in water media. Bioorganic Chem. 2018, 77, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Shi, H. Aqueous Foams in the Presence of Surfactant Crystals. Langmuir 2020, 36, 991–1002. [Google Scholar] [CrossRef]
- Artusio, F.; Bazzano, M.; Pisano, R.; Coulon, P.; Rizza, G.; Schiller, T.; Sangermano, M. Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer 2018, 139, 155–162. [Google Scholar] [CrossRef]
- Schröder, A.; Berton-Carabin, C.; Venema, P.; Cornacchia, L. Interfacial properties of whey protein and whey protein hydrolysates and their influence on O/W emulsion stability. Food Hydrocoll. 2017, 73, 129–140. [Google Scholar] [CrossRef]
- Abismäil, B.; Canselier, J.P.; Wilhelm, A.M.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Dheeraj Kumar Singh, B.V.V.S. Communication Reversible control of pore size and surface chemistry of mesoporous silica through dynamic covalent chemistry: Philicity mediated catalysis. Nanoscale 2015, 7, 13358–13362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Wang, T.; Fan, Y.; Kang, X.; Jia, R.; Kenzhebek, I.; Issakhov, M. Fabrication of a pH-responsive emulsifier for heavy oil recovery based on dynamic imine bond. J. Mol. Liq. 2021, 332, 115916. [Google Scholar] [CrossRef]













| Oil | Liquid Paraffin | n-Tetradecane | Toluene | Dichloromethane |
|---|---|---|---|---|
| Dielectric constant | 1.9 | 2.03 | 2.4 | 9.1 |
| Viscosity (cP, 20 °C) | 40 | 1.37 | 0.59 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Li, B.; Ren, L.; Lu, D.; Zhang, P.; Tian, L.; Di, W.; Shao, W.; He, J.; Sun, D. pH-Responsive Nanoemulsions Based on a Dynamic Covalent Surfactant. Nanomaterials 2021, 11, 1390. https://doi.org/10.3390/nano11061390
Ren G, Li B, Ren L, Lu D, Zhang P, Tian L, Di W, Shao W, He J, Sun D. pH-Responsive Nanoemulsions Based on a Dynamic Covalent Surfactant. Nanomaterials. 2021; 11(6):1390. https://doi.org/10.3390/nano11061390
Chicago/Turabian StyleRen, Gaihuan, Bo Li, Lulu Ren, Dongxu Lu, Pan Zhang, Lulu Tian, Wenwen Di, Weili Shao, Jianxin He, and Dejun Sun. 2021. "pH-Responsive Nanoemulsions Based on a Dynamic Covalent Surfactant" Nanomaterials 11, no. 6: 1390. https://doi.org/10.3390/nano11061390
APA StyleRen, G., Li, B., Ren, L., Lu, D., Zhang, P., Tian, L., Di, W., Shao, W., He, J., & Sun, D. (2021). pH-Responsive Nanoemulsions Based on a Dynamic Covalent Surfactant. Nanomaterials, 11(6), 1390. https://doi.org/10.3390/nano11061390