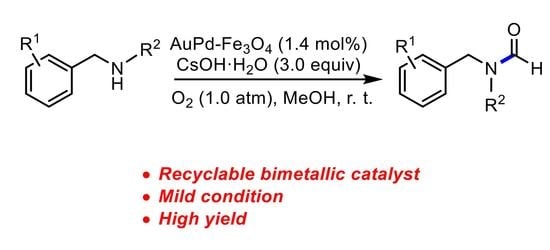
Oxidative N-Formylation of Secondary Amines Catalyzed by Reusable Bimetallic AuPd–Fe3O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Catalyst Characterization
3.2. Reaction Optimization
3.3. Effect of Alloy Bimetallic Catalyst vs. Combination of Two Metal Catalysts
3.4. Substrate Scope
3.5. Mechanistic Investigation
3.6. Effect of Au:Pd Ratio and Various Supports
3.7. Recycling Test
3.8. Gram Scale Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gerack, C.J.; McElwee-White, L. Formylation of Amines. Molecules 2014, 19, 7689–7713. [Google Scholar] [CrossRef] [Green Version]
- Schardl, C.L.; Grossman, R.B.; Nagabhyru, P.; Faulkner, J.R.; Mallik, U.P. Loline alkaloids: Currencies of mutualism. Phytochemistry 2007, 68, 980–996. [Google Scholar] [CrossRef]
- Thauer, R.K. Biochemistry of methanogenesis: A tribute to Marjory Stephenson. Microbiology 1998, 144, 2377–2406. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Takagi, H.; Minakami, H.; Hamanaka, W.; Okamoto, K.; Ito, A.; Sahashi, Y. A New Thiamine Derivative, S-Benzoylthiamine O-Monophosphate. Science 1961, 134, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Forsch, R.A.; Rosowsky, A. A New One-Step Synthesis of Leucovorin from Folic Acid and of 5-Formyl-5,6,7,8-tetrahydrohomofolic Acid from Homofolic Acid Using Dimethylamine-Borane in Formic Acid. J. Org. Chem. 1985, 50, 2582–2583. [Google Scholar] [CrossRef]
- Ravina, E. The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs; Wiley-VCH: Weinheim, Germany, 2011; pp. 157–159. [Google Scholar]
- Lonsdale, D. Thiamine tetrahydrofurfuryl disulfide: A little known therapeutic agent. Med. Sci. Monit. 2004, 10, RA199–RA203. [Google Scholar] [PubMed]
- Olah, G.A.; Ohannesian, L.; Arvanaghi, M. Formylating Agents. Chem. Rev. 1987, 87, 671–686. [Google Scholar] [CrossRef]
- Bao, K.; Zhang, W.; Bu, X.; Song, Z.; Zhang, L.; Cheng, M. A novel type of N-formylation and related reactions of amines via cyanides and esters as formylating agents. Chem. Commun. 2008, 42, 5429–5431. [Google Scholar] [CrossRef]
- Ke, Z.; Zhang, Y.; Cui, X.; Shi, F. Supported nano-gold-catalyzed N-formylation of amines with paraformaldehyde in water under ambient conditions. Green Chem. 2016, 18, 808–816. [Google Scholar] [CrossRef]
- Blicke, F.F.; Lu, C.-J. Formylation of Amines with Chloral and Reduction of the N-Formyl Derivatives with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1952, 74, 3933–3934. [Google Scholar] [CrossRef]
- Yale, H.L. Formylation of Amines with Phenyl Formate. J. Org. Chem. 1971, 36, 3238–3240. [Google Scholar] [CrossRef]
- Djuric, S.W. A Mild and Convenient Procedure for the N-Formylation of Secondary Amines Using Organosilicon Chemistry. J. Org. Chem. 1984, 49, 1311–1312. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Sharghi, H. ZnO as a New Catalyst for N-Formylation of Amines under Solvent-Free Conditions. J. Org. Chem. 2006, 71, 6652–6654. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.G.; Kumar, G.D.K.; Baskaran, S. A convenient method for the N-formylation of secondary amines and anilines using ammonium formate. Tetrahedron Lett. 2000, 41, 9149–9151. [Google Scholar] [CrossRef]
- Bhojegowd, M.R.M.; Nizam, A.; Pasha, M.A. Amberlite IR-120: A Reusable Catalyst for N-Formylation of Amines with Formic Acid Using Microwaves. Chin. J. Catal. 2010, 31, 518–520. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Lu, M. Highly efficient N-formylation of amines with ammonium formate catalyzed by nano-Fe3O4 in PEG-400. RSC Adv. 2014, 4, 1234–1240. [Google Scholar] [CrossRef]
- Keim, W. C1 Chemistry: Potential and developments. Pure Appl. Chem. 1986, 58, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Natte, K.; Neumann, H.; Beller, M.; Jagadeesh, R.V. Transition-Metal-Catalyzed Utilization of Methanol as a C1 Source in Organic Synthesis. Angew. Chem. Int. Ed. 2017, 56, 6384–6394. [Google Scholar] [CrossRef]
- Grigg, R.; Mitchell, T.R.B.; Sutthivaiyakit, S.; Tongpenyai, N. Transition Metal-catalysed N-Alkylation of Amines by Alcohols. J. Chem. Soc. Chem. Commun. 1981, 12, 611–612. [Google Scholar] [CrossRef]
- Dang, T.T.; Ramalingam, B.; Seayad, A.M. Efficient Ruthenium-Catalyzed N-Methylation of Amines Using Methanol. ACS Catal. 2015, 5, 4082–4088. [Google Scholar] [CrossRef]
- Elangovan, S.; Neumann, J.; Sortais, J.-B.; Junge, K.; Darcel, C.; Beller, M. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 2016, 7, 12641–12648. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y. Recent Advances in Methylation: A Guide for Selecting Methylation Reagents. Chem. Eur. J. 2019, 25, 3405–3439. [Google Scholar] [CrossRef]
- Meng, C.; Liu, P.; Tung, N.T.; Han, X.; Li, F. N-Methylation of Amines with Methanol in Aqueous Solution Catalyzed by a Water-Soluble Metal–Ligand Bifunctional Dinuclear Iridium Catalyst. J. Org. Chem. 2020, 85, 5815–5824. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Wei, Z.; Zhai, Y.; Ru, S.; Zhao, Q.; Wang, J.; Han, S.; Wei, Y. N-formylation of amines using methanol as a potential formyl carrier by a reusable chromium catalyst. Commun. Chem. 2019, 2, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Motahharifar, N.; Sajjadi, M.; Aghbolagh, A.M.; Shokouhimehr, M.; Varma, R.S. Recent advances in N-formylation of amines and nitroarenes using efficient (nano)catalysts in ecofriendly media. Green Chem. 2019, 21, 5144–5167. [Google Scholar] [CrossRef]
- Pichardo, M.C.; Tavakoli, G.; Armstrong, J.E.; Wilczek, T.; Thomas, B.E.; Prechtl, M.H.G. Copper-Catalyzed Formylation of Amines by using Methanol as the C1 Source. ChemSusChem 2020, 13, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankar, S.; Neumann, H.; Beller, M. A Convenient and Practical Synthesis of Anisoles and Deuterated Anisoles by Palladium-Catalyzed Coupling Reactions of Aryl Bromides and Chlorides. Chem. Eur. J. 2012, 18, 2498–2502. [Google Scholar] [CrossRef]
- Dash, P.; Janni, M.; Peruncheralathan, S. Trideuteriomethoxylation of Aryl and Heteroaryl Halides. Eur. J. Org. Chem. 2012, 26, 4914–4917. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [Green Version]
- Cole-Hamilton, D.J. Homogeneous Catalysis—New Approaches to Catalyst Separation, Recovery, and Recycling. Science 2003, 299, 1702–1706. [Google Scholar] [CrossRef]
- Hartwig, J.F.; Collman, J.P. Organotransition Metal Chemistry: From Bonding to Catalysis; University Science Books: Sausalito, CA, USA, 2010; pp. 546–549. [Google Scholar]
- Crabtree, R.H. Resolving Heterogeneity Problems and Impurity Artifacts in Operationally Homogeneous Transition Metal Catalysts. Chem. Rev. 2012, 112, 1536–1554. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Richter, C.; Glorius, F. N-Formylation of Amines by Methanol Activation. Org. Lett. 2013, 15, 1776–1779. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gellrich, U.; Diskin-Posner, Y.; Leitus, G.; Avram, L.; Milstein, D. Manganese-Catalyzed N-Formylation of Amines by Methanol Liberating H2: A Catalytic and Mechanistic Study. Angew. Chem. Int. Ed. 2017, 56, 4229–4233. [Google Scholar] [CrossRef]
- Kang, B.; Hong, S.H. Hydrogen Acceptor- and Base-Free N-Formylation of Nitriles and Amines using Methanol as C1 Source. Adv. Synth. Catal. 2015, 357, 834–840. [Google Scholar] [CrossRef]
- Choi, G.; Hong, S.H. Selective N-Formylation and N-Methylation of Amines Using Methanol as a Sustainable C1 Source. ACS Sustain. Chem. Eng. 2019, 7, 716–723. [Google Scholar] [CrossRef]
- Shekhar, A.C.; Kumar, A.R.; Sathaiah, G.; Paul, V.L.; Sridhar, M.; Rao, P.S. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009, 50, 7099–7101. [Google Scholar] [CrossRef]
- Pathare, S.P.; Sawant, R.V.; Akamanchi, K.G. Sulfated tungstate catalyzed highly accelerated N-formylation. Tetrahedron Lett. 2012, 53, 3259–3263. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Rahimizadeh, M.; Moeinpour, F.; Eshghi, H.; Bakavoli, M. Polyphosphoric acid supported on silica-coated NiFe2O4 nanoparticles: An efficient and magnetically-recoverable catalyst for N-formylation of amines. C. R. Chim. 2014, 17, 459–464. [Google Scholar] [CrossRef]
- Kooti, M.; Nasiri, E. Phosphotungstic acid supported on silica-coated CoFe2O4 nanoparticles: An efficient and magnetically-recoverable catalyst for N-formylation of amines under solvent-free conditions. J. Mol. Catal. A Chem. 2015, 406, 168–177. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Verma, S.; Nadagouda, M.N.; Varma, R.S. A photoactive bimetallic framework for direct aminoformylation of nitroarenes. Green Chem. 2016, 18, 1019–1022. [Google Scholar] [CrossRef]
- Luo, R.; Lin, X.; Chen, Y.; Zhang, W.; Zhou, X.; Ji, H. Cooperative Catalytic Activation of Si–H Bonds: CO2-Based Synthesis of Formamides from Amines and Hydrosilanes under Mild Conditions. ChemSusChem 2017, 10, 1224–1232. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, X.; Zhou, S.; Yang, P.; Du, C.-X.; Li, Y. Mn-Catalyzed Selective Double and Mono-N-Formylation and N-Methylation of Amines by using CO2. ChemSusChem 2019, 12, 3054–3059. [Google Scholar] [CrossRef] [Green Version]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.-M. Magnetically Recoverable Nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Au-Pd/TiO2 Catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef]
- Hou, W.; Dehm, N.A.; Scott, R.W.J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts. J. Catal. 2008, 253, 22–27. [Google Scholar] [CrossRef]
- Chang, C.-R.; Long, B.; Yang, X.-F.; Li, J. Theoretical Studies on the Synergetic Effects of Au–Pd Bimetallic Catalysts in the Selective Oxidation of Methanol. J. Phys. Chem. C 2015, 119, 16072–16081. [Google Scholar] [CrossRef]
- Han, S.; Mullins, C.B. Surface Alloy Composition Controlled O2 Activation on Pd–Au Bimetallic Model Catalysts. ACS Catal. 2018, 8, 3641–3649. [Google Scholar] [CrossRef]
- Preedasuriyachai, P.; Kitahara, H.; Chavasiri, W.; Sakurai, H. N-Formylation of Amines Catalyzed by Nanogold under Aerobic Oxidation Conditions with MeOH or Formalin. Chem. Lett. 2010, 39, 1174–1176. [Google Scholar] [CrossRef]
- Ishida, T.; Haruta, M. N-Formylation of Amines via the Aerobic Oxidation of Methanol over Supported Gold Nanoparticles. ChemSusChem 2009, 2, 538–541. [Google Scholar] [CrossRef]
- Tumma, H.; Nagaraju, N.; Reddy, K.V. A facile method for the N-formylation of primary and secondary amines by liquid phase oxidation of methanol in the presence of hydrogen peroxide over basic copper hydroxyl salts. J. Mol. Catal. A 2009, 310, 121–129. [Google Scholar] [CrossRef]
- Tanaka, S.; Minato, T.; Ito, E.; Hara, M.; Kim, Y.; Yamamoto, Y.; Asao, N. Selective Aerobic Oxidation of Methanol in the Coexistence of Amines by Nanoporous Gold Catalysts: Highly Efficient Synthesis of Formamides. Chem. Eur. J. 2013, 19, 11832–11836. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, J.; Kim, S.; Jun, S.W.; Kim, B.H.; Lee, D.W.; Kim, B.M.; Hyeon, T. Simple synthesis of Pd–Fe3O4 heterodimer nanocrystals and their application as a magnetically recyclable catalyst for Suzuki cross-coupling reactions. Phys. Chem. Chem. Phys. 2011, 13, 2512–2516. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.; Jun, S.W.; Kim, B.H.; Hwang, S.; Song, I.K.; Kim, B.M.; Hyeon, T. Simple one-pot synthesis of Rh–Fe3O4 heterodimer nanocrystals and their applications to a magnetically recyclable catalyst for efficient and selective reduction of nitroarenes and alkenes. Chem. Commun. 2011, 47, 3601–3603. [Google Scholar] [CrossRef]
- Lee, J.; Chung, J.; Byun, S.; Kim, B.M.; Lee, C. Direct catalytic C–H arylation of imidazo [1,2-a]pyridine with aryl bromides using magnetically recyclable Pd–Fe3O4 nanoparticles. Tetrahedron 2013, 69, 5660–5664. [Google Scholar] [CrossRef]
- Chung, J.; Kim, J.; Jang, Y.; Byun, S.; Hyeon, T.; Kim, B.M. Heck and Sonogashira cross-coupling reactions using recyclable Pd–Fe3O4 heterodimeric nanocrystal catalysts. Tetrahedron Lett. 2013, 54, 5192–5196. [Google Scholar] [CrossRef]
- Byun, S.; Chung, J.; Jang, Y.; Kwon, J.; Hyeon, T.; Kim, B.M. Highly selective Wacker oxidation of terminal olefins using magnetically recyclable Pd–Fe3O4 heterodimer nanocrystals. RSC Adv. 2013, 3, 16296–16299. [Google Scholar] [CrossRef]
- Bae, I.H.; Lee, I.-H.; Byun, S.; Chung, J.; Kim, B.M.; Choi, T.-L. Magnetically Recyclable Pd-Fe3O4 Heterodimer Nanocrystals for the Synthesis of Conjugated Polymers via Suzuki Polycondensation: Toward Green Chemistry. J Polym. Sci. Pol. Chem. 2014, 52, 1525–1528. [Google Scholar] [CrossRef]
- Kwon, J.; Chung, J.; Byun, S.; Kim, B.M. Efficient Synthesis of Indole Derivatives via Tandem Cyclization Catalyzed by Magnetically Recoverable Palladium/Magnetite (Pd-Fe3O4) Nanocrystals. Asian J. Org. Chem. 2016, 5, 470–476. [Google Scholar] [CrossRef]
- Byun, S.; Song, Y.; Kim, B.M. Heterogenized Bimetallic Pd–Pt–Fe3O4 Nanoflakes as Extremely Robust, Magnetically Recyclable Catalysts for Chemoselective Nitroarene Reduction. ACS Appl. Mater. Interfaces 2016, 8, 14637–14647. [Google Scholar] [CrossRef]
- Jang, J.; Byun, S.; Kim, B.M.; Lee, S. Arylsilylation of aryl halides using the magnetically recyclable bimetallic Pd–Pt–Fe3O4 catalyst. Chem. Commun. 2018, 54, 3492–3495. [Google Scholar] [CrossRef]
- Cho, A.; Byun, S.; Kim, B.M. AuPd–Fe3O4 Nanoparticle Catalysts for Highly Selective, One-Pot Cascade Nitro-Reduction and Reductive Amination. Adv. Synth. Catal. 2018, 360, 1253–1261. [Google Scholar] [CrossRef]
- Cho, A.; Byun, S.; Cho, J.H.; Kim, B.M. AuPd–Fe3O4 Nanoparticle-Catalyzed Synthesis of Furan-2,5-dimethylcarboxylate from 5-Hydroxymethylfurfural under Mild Conditions. ChemSusChem 2019, 12, 2310–2317. [Google Scholar] [CrossRef]
- Cho, J.H.; Byun, S.; Cho, A.; Kim, B.M. One-pot, chemoselective synthesis of secondary amines from aryl nitriles using a PdPt–Fe3O4 nanoparticle catalyst. Catal. Sci. Technol. 2020, 10, 4201–4209. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Chen, C.; Qin, Z.; Zhu, H.; Wang, G.; Wang, H.; Wu, C.; Dong, W.; Fan, W.; et al. Graphene-supported Au–Pd bimetallic nanoparticles with excellent catalytic performance in selective oxidation of methanol to methyl formate. Chem. Commun. 2013, 49, 8250–8252. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Q.; Sun, Y.; Chen, S.; Wang, J.-Q.; Wu, M.; Fu, W.; Tang, Y.; Duan, X.; Chen, D.; et al. Optimising surface d charge of AuPd nanoalloy catalysts for enhanced catalytic activity. Nat. Commun. 2019, 10, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Ju, P.; Chen, J.; Chen, A.; Chen, L.; Yu, Y. N-Formylation of Amines with CO2 and H2 Using Pd–Au Bimetallic Catalysts Supported on Polyaniline-Functionalized Carbon Nanotubes. ACS Sustain. Chem. Eng. 2017, 5, 2516–2528. [Google Scholar]
- Wang, R.; Wu, Z.; Wang, G.; Qin, Z.; Chen, C.; Dong, M.; Zhu, H.; Fan, W.; Wang, J. Highly active Au–Pd nanoparticles supported on three-dimensional graphene–carbon nanotube hybrid for selective oxidation of methanol to methyl formate. RSC Adv. 2015, 5, 44835–44839. [Google Scholar] [CrossRef]
- Beller, M. The Current Status and Future Trends in Oxidation Chemistry. Adv. Synth. Catal. 2004, 346, 107–108. [Google Scholar] [CrossRef]
- Harit, H.; Hiran, B.L.; Joshi, S.N. Kinetics and Mechanism of Oxidation of Pimary Alcohols by Pyridinium Dichromate. Chem. Sci. Trans. 2015, 4, 49–58. [Google Scholar]
- Jana, S.; Thomas, J.; Gupta, S.S. Catalytic oxidation of alcohols using Fe-bTAML and NaClO: Comparing the reactivity of Fe(V)O and Fe(IV)O intermediates. Inorg. Chim. Acta 2019, 486, 476–482. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef]
- Camellone, M.F.; Marx, D. Nature and Role of Activated Molecular Oxygen Species at the Gold/Titania Interface in the Selective Oxidation of Alcohols. J. Phys. Chem. C 2014, 118, 20989–21000. [Google Scholar] [CrossRef]
- Whiting, G.T.; Kondrat, S.A.; Hammond, C.; Dimitratos, N.; He, Q.; Morgan, D.J.; Dummer, N.F.; Bartley, J.K.; Kiely, C.J.; Taylor, S.H.; et al. Methyl Formate Formation from Methanol Oxidation Using Supported Gold–Palladium Nanoparticles. ACS Catal. 2015, 5, 637–644. [Google Scholar] [CrossRef]
- Tong, X.; Liu, Z.; Yu, L.; Li, Y. A tunable process: Catalytic transformation of renewable furfural with aliphatic alcohols in the presence of molecular oxygen. Chem. Commun. 2015, 51, 3674–3677. [Google Scholar] [CrossRef]
- Slot, T.K.; Eisenberg, D.; van Noordenne, D.; Jungbacker, P.; Rothenberg, G. Cooperative Catalysis for Selective Alcohol Oxidation with Molecular Oxygen. Chem. Eur. J. 2016, 22, 12307–12311. [Google Scholar] [CrossRef]
- Ray, R.; Chandra, S.; Maiti, D.; Lahiri, G.K. Simple and Efficient Ruthenium-Catalyzed Oxidation of Primary Alcohols with Molecular Oxygen. Chem. Eur. J. 2016, 22, 8814–8822. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705–13725. [Google Scholar] [CrossRef]
- Singh, A.K.; Xu, Q. Synergistic Catalysis over Bimetallic Alloy Nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Ma, Y.; Zhang, H.; Gao, J.; Nie, Y.; Sun, X. Aqueous Solution Synthesis of Pt–M (M = Fe, Co, Ni) Bimetallic Nanoparticles and Their Catalysis for the Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2014, 6, 12429–12435. [Google Scholar] [CrossRef]
- Yu, H.; Tang, W.; Li, K.; Zhao, S.; Yin, H.; Zhou, S. Enhanced Catalytic Performance for Hydrogenation of Substituted Nitroaromatics over Ir-Based Bimetallic Nanocatalysts. ACS Appl. Mater. Interfaces 2019, 11, 6958–6969. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Highly Efficient Catalysts of Bimetallic Pt–Ru Nanocrystals Supported on Ordered ZrO2 Nanotube for Toluene Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 13781–13789. [Google Scholar] [CrossRef]










| Entry | Catalyst | Base | TON 2 | Yield 3 |
|---|---|---|---|---|
| 1 | Fe3O4 | NaOH | 0 | N. D. 4 |
| 2 5 | Pd–Fe3O4 | NaOH | 20 | 55 |
| 3 6 | Au–Fe3O4 | NaOH | 9 | 25 |
| 4 | AuPd–Fe3O4 | NaOH | 23 | 65 |
| 5 | AuPd–Fe3O4 | - | 5 | 14 |
| 6 7 | AuPd–Fe3O4 | NaOH | 15 | 43 |
| 7 | AuPd–Fe3O4 | Cs2CO3 | 25 | 69 |
| 8 | AuPd–Fe3O4 | CsOH·H2O | 33 | 90 8 |
| 9 9 | AuPd–Fe3O4 | CsOH·H2O | 33 | 92 (84 10) |
| 10 11 | AuPd–Fe3O4 | CsOH·H2O | 32 | 89 |
| 11 12 | AuPd–Fe3O4 | CsOH·H2O | 15 | 42 13 |
| 12 14 | AuPd–Fe3O4 | CsOH·H2O | 89 | 71 |

| Entry | Base | Yield 2 | Entry | Base | Yield 2 |
|---|---|---|---|---|---|
| 1 | - | 14 | 6 | K2CO3 | 54 |
| 2 | LiOH·H2O | 60 | 7 | Cs2CO3 | 69 |
| 3 | NaOH | 65 | 8 | KOt Bu | 69 |
| 4 | KOH | 75 | 9 | K3PO4 | 60 |
| 5 | CsOH·H2O | 91 | 10 | CsF | 39 |

| Entry | Catalyst 3 | Product Yield with Various R Group (%) 2 | ||||
|---|---|---|---|---|---|---|
| H | 4-Me | 4-OMe | 3-NO2 | 3-CN | ||
| 1 4 | Au | 51 | 61 | 27 | 25 | 23 |
| 2 5 | Pd | 57 | 28 | 18 | 8 | 5 |
| 3 6 | Au + Pd | 90 | 62 | 34 | 26 | 32 |
| 4 7 | AuPd | 91 | 69 | 83 | 73 | 59 |

| Entry | Catalyst | Yield 2 |
|---|---|---|
| 1 | Au1Pd0.23–Fe3O4 | 76 |
| 2 | Au1Pd0.39–Fe3O4 | 76 |
| 3 | Au1Pd1.08–Fe3O4 | 91 |
| 4 | Au0.65Pd1–Fe3O4 | 79 |
| 5 | Au0.39Pd1–Fe3O4 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Cho, A.; Cho, J.H.; Kim, B.M. Oxidative N-Formylation of Secondary Amines Catalyzed by Reusable Bimetallic AuPd–Fe3O4 Nanoparticles. Nanomaterials 2021, 11, 2101. https://doi.org/10.3390/nano11082101
Yang S, Cho A, Cho JH, Kim BM. Oxidative N-Formylation of Secondary Amines Catalyzed by Reusable Bimetallic AuPd–Fe3O4 Nanoparticles. Nanomaterials. 2021; 11(8):2101. https://doi.org/10.3390/nano11082101
Chicago/Turabian StyleYang, Sabyuk, Ahra Cho, Jin Hee Cho, and Byeong Moon Kim. 2021. "Oxidative N-Formylation of Secondary Amines Catalyzed by Reusable Bimetallic AuPd–Fe3O4 Nanoparticles" Nanomaterials 11, no. 8: 2101. https://doi.org/10.3390/nano11082101
APA StyleYang, S., Cho, A., Cho, J. H., & Kim, B. M. (2021). Oxidative N-Formylation of Secondary Amines Catalyzed by Reusable Bimetallic AuPd–Fe3O4 Nanoparticles. Nanomaterials, 11(8), 2101. https://doi.org/10.3390/nano11082101