Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
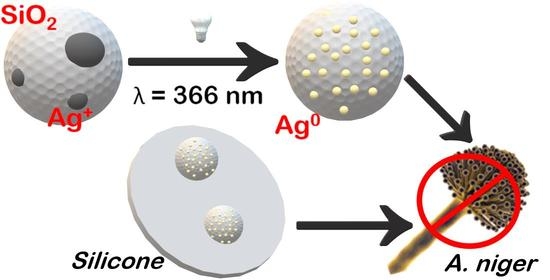
2.2. Preparation of SiO2-Ag Samples
2.3. Preparation of Silicone-Composite Samples
2.4. Characterization
2.5. Antifungal and Antibiofilm Tests
3. Results
3.1. Material Preparation
3.2. Characterization of Materials
3.3. Antifungal Activity
3.4. Preparation and Characterization of Silicone-Composites
3.5. Biofilm Activity of Silicone-Composite Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metzger, J.B.; Garagusi, V.F.; Kerwin, D.M. Pulmonary oxalosis caused by Aspergillus niger. Am. J. Respir. Crit Care Med. 1984, 129, 501–502. [Google Scholar]
- Liev, I.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.; Van Dijck, P. On the Safety of Aspergillus niger—A Review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Self-disinfecting surfaces: Review of current methodologies and future prospects. Am. J. Infect. Control 2013, 41, S31–S35. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Yang, W.; Gao, S.; Sun, C.; Li, Q. Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 2019, 1, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Ambrogi, V.; Pietrella, D.; Donnadio, A.; Latterini, L.; Di Michele, A.; Luffarelli, I.; Ricci, M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C 2020, 112, 110863. [Google Scholar] [CrossRef]
- Ambrogi, V.; Donnadio, A.; Pietrella, D.; Latterini, L.; Proietti, F.A.; Marmottini, F.; Padeletti, G.; Kaciulis, S.; Giovagnoli, S.; Ricci, M. Chitosan Films Containing Mesoporous SBA-15 Supported Silver Nanoparticles for Wound Dressing. J. Mater. Chem. B 2014, 2, 6054–6063. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Fortunati, E.; Taddei, M.; Kenny, J.M.; Armentano, I.; Latterini, L. Integrated PLGA-Ag Nanocomposite Systems to Control the Degradation Rate and Antibacterial Properties. J. Appl. Polym. Sci. 2013, 130, 1185–1193. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interf. Sci. 2020, 284, 102246. [Google Scholar] [CrossRef] [PubMed]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver Particles in Medical Applications: Synthesis, Performance, and Toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yu, S.H.; Wang, C.Y.; Li, X.G.; Zhu, Y.R.; Chen, Z.Y. A Novel ultraviolet irradiation photoreduction technique for the preparation of single- crystal Ag nanorods and Ag dendrites. Adv. Mat. 1999, 11, 850–852. [Google Scholar] [CrossRef]
- Sato-Berrú, R.; Redón, R.; Vázquez-Olmos, A.; Saniger, J. Silver nanoparticles synthesized by direct photoreduction of metal salts. Application in surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 376–380. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced Conversion of Silver Nanospheres to Nanoprisms. Science 2001, 294, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS-a Single-Molecule and Nanoscale Tool for Bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef]
- De Cremer, G.; Antoku, Y.; Roeffaers, M.B.J.; Sliwa, M.; Van Noyen, J.; Smout, S.; Hofkens, J.; De Vos, D.E.; Sels, B.F.; Vosch, T. Photoactivation of Silver-Exchanged Zeolite A. Angew. Chem. Int. 2008, 47, 2813–2816. [Google Scholar] [CrossRef]
- Meenakshi, S.; Devi, S.; Pandian, K.; Devendiran, R.; Selvaraj, M. Sunlight Assisted Synthesis of Silver Nanoparticles in Zeolite Matrix and Study of Its Application on Electrochemical Detection of Dopamine and Uric Acid in Urine Samples. Mater. Sci. Eng. C 2016, 69, 85–94. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lin, S.X.; Weng, C.F.; Lee, C.H. pH-triggered controllable release of silver–indole-3 acetic acid complexes from mesoporous silica nanoparticles (IBN-4) for effectively killing malignant bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.D.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos. Sci. Technol. 2008, 68, 2948–2953. [Google Scholar] [CrossRef]
- Fenwick, O.; Coutiño-Gonzalez, E.; Grandjean, D.; Baekelant, W.; Richard, F.; Bonacchi, S.; De Vos, D.; Lievens, P.; Roeffaers, M.; Hofkens, J.; et al. Tuning the Energetics and Tailoring the Optical Properties of Silver Clusters Confined in Zeolites. Nat. Mater. 2016, 15, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Excipient, P. Syloid ® Fp. Available online: https://www.grace.com/materials-technologies (accessed on 2 June 2021).
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal Activity and Mode of Action of Silver Nano-Particles on Candida Albicans. BioMetals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- De Buyl, F. Silicone sealants and structural adhesives. IJAA 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Kottmann, A.; Mejìa, E.; Hèmery, T.; Klein, J.; Kragl, U. Recent developments in the preparation of silicones with antimicrobial properties. Chem. Asian J. 2017, 12, 1168–1179. [Google Scholar] [CrossRef]
- Klosowski, J.M. Sealants in Construction; Marcel Dekker Inc.: New York, NY, USA, 1989; Chapter 6. [Google Scholar]
- Ghamrawi, S.; Bouchara, J.P.; Corbin, A.; Rogalsky, S.; Tarasyuk, O.; Bardeau, J.F. Inhibition of fungal growth by silicones modified with cationic biocides. Mater. Today Commun. 2020, 22, 1007166. [Google Scholar] [CrossRef]
- He, C.; Liu, L.; Fang, Z.; Li, J.; Guo, J.; Wei, J. Formation and characterization of silver nanoparticles in aqueous solution via ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 542–548. [Google Scholar] [CrossRef]
- Allen, J.P.; Scanlon, D.O.; Watson, W.G. Electron structure of silver oxides. Phys. Rev. B 2011, 84, 115141. [Google Scholar] [CrossRef]
- Li, Z.; Nyalosaso, J.L.; Hwang, A.A.; Ferris, D.P.; Yang, S.; Derrien, G.; Charnay, C.; Durand, J.O.; Zink, J.I. Measurement of Uptake and Release Capacities of Mesoporous Silica Nanoparticles Enabled by Nanovalve Gates. J. Phys. Chem. C 2011, 115, 19496–19506. [Google Scholar] [CrossRef] [Green Version]
- Tarpani, L.; Ruhlandt, D.; Latterini, L.; Haehnel, D.; Gregor, I.; Enderlein, J.; Chizhik, A.I. Photoactivation of Luminescent Centers in Single SiO2 nanoparticles. Nano Lett. 2016, 16, 4312–4316. [Google Scholar] [CrossRef]
- Spallino, L.; Vaccaro, L.; Agnello, S.; Cannas, M. Effects induced by UV laser radiation on the blue luminescence of silica nanoparticles. J. Lumin. 2013, 138, 39–43. [Google Scholar] [CrossRef]
- Spallino, L.; Spera, M.; Vaccaro, L.; Agnello, S.; Gelardi, F.M.; Zatsepin, A.F.; Cannas, M. Environment assisted photoconversion of luminescent surface defects in SiO2 nanoparticles. Appl. Surf. Sci. 2017, 420, 94–99. [Google Scholar] [CrossRef]
- Selvaggi, R.; Tarpani, L.; Santuari, A.; Giovagnoli, S.; Latterini, L. Silica nanoparticles assisted photodegradation of acridine orange in aqueous suspensions. Appl. Catal. B 2015, 168, 363–369. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sułowicz, S.; Nowak, B.; Piotrowska-Segret, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef] [Green Version]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, copper, and copper hydroxy salt decorated fumed silica hybrid composites as antibacterial agents. Colloids Surf. B 2020, 195, 111216. [Google Scholar] [CrossRef] [PubMed]
- Molleman, B.; Hiemstra, T. Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir 2015, 31, 13361–13372. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]




| Sample | Ag (ppm) | % Ag in the Sample | Zeta Potential (mV) |
|---|---|---|---|
| SiO2 | −93.50 | ||
| SiO2-Ag | 2.98 | 0.62% | 11.45 |
| SiO2-Ag-Irr | 3.09 | 0.66% | −69.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaglia, G.; Ambrogi, V.; Pietrella, D.; Nocchetti, M.; Latterini, L. Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity. Nanomaterials 2021, 11, 2340. https://doi.org/10.3390/nano11092340
Quaglia G, Ambrogi V, Pietrella D, Nocchetti M, Latterini L. Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity. Nanomaterials. 2021; 11(9):2340. https://doi.org/10.3390/nano11092340
Chicago/Turabian StyleQuaglia, Giulia, Valeria Ambrogi, Donatella Pietrella, Morena Nocchetti, and Loredana Latterini. 2021. "Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity" Nanomaterials 11, no. 9: 2340. https://doi.org/10.3390/nano11092340
APA StyleQuaglia, G., Ambrogi, V., Pietrella, D., Nocchetti, M., & Latterini, L. (2021). Solid State Photoreduction of Silver on Mesoporous Silica to Enhance Antifungal Activity. Nanomaterials, 11(9), 2340. https://doi.org/10.3390/nano11092340