Surface Charge Transfer Doping of MoS2 Monolayer by Molecules with Aggregation-Induced Emission Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. CVD Growth of MoS2
2.2. Synthesis of AIE Molecules
2.3. Characterizations
2.4. DFT Calculation
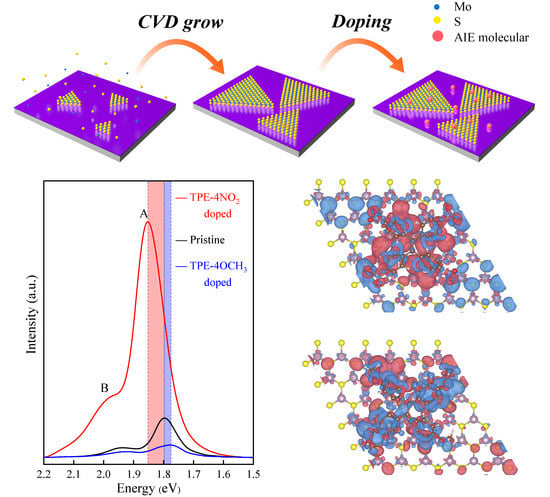
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, K.F.; He, K.L.; Shan, J.; Heinz, T.F. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nanotechnol. 2012, 7, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, G.; Han, W.P.; Ye, H.Q.; Zhu, C.R.; Shi, J.R.; Niu, Q.; Tan, P.H.; Wang, E.; Liu, B.L.; et al. Valley-selective circular dichroism of monolayer molybdenum disulphide. Nat. Commun. 2012, 3, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baugher, B.W.H.; Churchill, H.O.H.; Yang, Y.F.; Jarillo-Herrero, P. Optoelectronic devices based on electrically tunable p-n diodes in a monolayer dichalcogenide. Nat. Nanotechnol. 2014, 9, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Radisavljevic, B.; Kis, A. Mobility engineering and a metal-insulator transition in monolayer MoS2. Nat. Mater. 2013, 12, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Buscema, M.; Groenendijk, D.J.; Steele, G.A.; van der Zant, H.S.J.; Castellanos-Gomez, A. Photovoltaic effect in few-layer black phosphorus PN junctions defined by local electrostatic gating. Nat. Commun. 2014, 5, 4651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.L.; Duan, X.D.; Wu, X.P.; Zhuang, X.J.; Zhou, H.; Zhang, Q.L.; Zhu, X.L.; Hu, W.; Ren, P.Y.; Guo, P.F.; et al. Growth of Alloy MoS2xSe2(1-x) Nanosheets with Fully Tunable Chemical Compositions and Optical Properties. J. Am. Chem. Soc. 2014, 136, 3756–3759. [Google Scholar] [CrossRef]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.Y.; Park, J.; Jung, H.J.; Chen, Y.B.; Ko, C.; Jang, C.; Sun, Y.H.; et al. Doping against the Native Propensity of MoS2: Degenerate Hole Doping by Cation Substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. New Route for Stabilization of 1T-WS2 and MoS2 Phases. J. Phys. Chem. C 2011, 115, 24586–24591. [Google Scholar] [CrossRef] [Green Version]
- Roy, T.; Tosun, M.; Cao, X.; Fang, H.; Lien, D.H.; Zhao, P.D.; Chen, Y.Z.; Chueh, Y.L.; Guo, J.; Javey, A. Dual-Gated MoS2/WSe2 van der Waals Tunnel Diodes and Transistors. ACS Nano 2015, 9, 2071–2079. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Han, C.; Chen, W. Surface charge transfer doping for two-dimensional semiconductor-based electronic and optoelectronic devices. Nano Res. 2021, 14, 1682–1697. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, Z.; Zhang, X.; He, Y.; Jie, J. Surface Charge Transfer Doping of Low-Dimensional Nanostructures toward High-Performance Nanodevices. Adv. Mater. 2016, 28, 10409–10442. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Zhou, J.; Ataca, C.; Liu, J.; Kang, J.S.; Matthews, T.S.; You, L.; Li, J.B.; Grossman, J.C.; Wu, J.Q. Broad-Range Modulation of Light Emission in Two-Dimensional Semiconductors by Molecular Physisorption Gating. Nano Lett. 2013, 13, 2831–2836. [Google Scholar] [CrossRef]
- Liu, B.L.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C.W. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Mono layer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]
- Fang, H.; Tosun, M.; Seol, G.; Chang, T.C.; Takei, K.; Guo, J.; Javey, A. Degenerate n-Doping of Few-Layer Transition Metal Dichalcogenides by Potassium. Nano Lett. 2013, 13, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable Photoluminescence of Monolayer MoS2 via Chemical Doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef] [Green Version]
- Kiriya, D.; Tosun, M.; Zhao, P.D.; Kang, J.S.; Javey, A. Air-Stable Surface Charge Transfer Doping of MoS2 by Benzyl Viologen. J. Am. Chem. Soc. 2014, 136, 7853–7856. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Hong, Y.N.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.J.; Lam, J.W.Y.; Tang, B.Z. Tetraphenylethene: A versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 23726–23740. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Yan, X.X.; Lu, Z.X.; Qiu, H.L.; Xu, G.C.; Xu, Z.; Peng, W.; Pan, X.Q.; Liu, K.H.; Jiao, L.Y. High-Mobility Multilayered MoS2 Flakes with Low Contact Resistance Grown by Chemical Vapor Deposition. Adv. Mater. 2017, 29, 1604540. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, X.; Kim, S.T.; Alahakoon, S.B.; Hou, X.; Zhang, Z.; Thompson, C.M.; Smaldone, R.A.; Ke, C. An Elastic Hydrogen-Bonded Cross-Linked Organic Framework for Effective Iodine Capture in Water. J. Am. Chem. Soc. 2017, 139, 7172–7175. [Google Scholar] [CrossRef]
- Mori, T.; Inoue, Y. Chiral Organic Radical Cation and Dication. A Reversible Chiroptical Redox Switch Based on Stepwise Transformation of Optically Active Tetrakis(p-alkoxyphenyl)ethylenes to Radical Cations and Dications. J. Phys. Chem. A 2005, 109, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Sandoval, S.J.; Divigalpitiya, W.M.R.; Irwin, J.C.; Frindt, R.F. Structure of single-molecular-layer MoS2. Phys. Rev. B 1991, 43, 12053–12056. [Google Scholar] [CrossRef]
- Aleithan, S.H.; Livshits, M.Y.; Khadka, S.; Rack, J.J.; Kordesch, M.E.; Stinaff, E. Broadband femtosecond transient absorption spectroscopy for a CVD MoS2 monolayer. Phys. Rev. B 2016, 94, 035445. [Google Scholar] [CrossRef] [Green Version]
- Nie, Z.; Long, R.; Teguh, J.S.; Huang, C.-C.; Hewak, D.W.; Yeow, E.K.L.; Shen, Z.; Prezhdo, O.V.; Loh, Z.-H. Ultrafast Electron and Hole Relaxation Pathways in Few-Layer MoS2. J. Phys. Chem. C 2015, 119, 20698–20708. [Google Scholar] [CrossRef]
- Wang, G.; Wang, K.; McEvoy, N.; Bai, Z.; Cullen, C.P.; Murphy, C.N.; McManus, J.B.; Magan, J.J.; Smith, C.M.; Duesberg, G.S.; et al. Ultrafast Carrier Dynamics and Bandgap Renormalization in Layered PtSe2. Small 2019, 15, 1902728. [Google Scholar] [CrossRef]
- Yarali, M.; Zhong, Y.R.; Reed, S.N.; Wang, J.F.; Ulman, K.A.; Charboneau, D.J.; Curley, J.B.; Hynek, D.J.; Pondick, J.V.; Yazdani, S.; et al. Near-Unity Molecular Doping Efficiency in Monolayer MoS2. Adv. Electron. Mater. 2021, 7, 2000873. [Google Scholar] [CrossRef]
- Huang, Y.; Zhuge, F.W.; Hou, J.X.; Lv, L.; Luo, P.; Zhou, N.; Gan, L.; Zhai, T.Y. Van der Waals Coupled Organic Molecules with Monolayer MoS2 for Fast Response Photodetectors with Gate-Tunable Responsivity. ACS Nano 2018, 12, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Lu, Z.Y.; Xu, S.C.; Kong, D.S.; Cha, J.J.; Zheng, G.Y.; Hsu, P.C.; Yan, K.; Bradshaw, D.; Prinz, F.B.; et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gali, S.M.; Slassi, A.; Beljonne, D.; Samori, P. Collective Dipole-Dominated Doping of Monolayer MoS2: Orientation and Magnitude Control via the Supramolecular Approach. Adv. Funct. Mater. 2020, 30, 2002846.1–2002846.10. [Google Scholar] [CrossRef]
- Obaidulla, S.M.; Habib, M.R.; Khan, Y.; Kong, Y.H.; Liang, T.; Xu, M.S. MoS2 and Perylene Derivative Based Type-II Heterostructure: Bandgap Engineering and Giant Photoluminescence Enhancement. Adv. Mater. Interfaces 2020, 7, 1901197. [Google Scholar] [CrossRef]
- Le, O.K.; Chihaia, V.; On, V.V.; Son, D.N. N-type and p-type molecular doping on monolayer MoS2. RSC Adv. 2021, 11, 8033–8041. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Sun, S.; Liang, X.; Gong, H.; Zhang, X.; Li, Y.; Gao, M.; Li, D.; Xu, G. Surface Charge Transfer Doping of MoS2 Monolayer by Molecules with Aggregation-Induced Emission Effect. Nanomaterials 2022, 12, 164. https://doi.org/10.3390/nano12010164
Sun R, Sun S, Liang X, Gong H, Zhang X, Li Y, Gao M, Li D, Xu G. Surface Charge Transfer Doping of MoS2 Monolayer by Molecules with Aggregation-Induced Emission Effect. Nanomaterials. 2022; 12(1):164. https://doi.org/10.3390/nano12010164
Chicago/Turabian StyleSun, Ruihao, Shiyu Sun, Xiu Liang, Hongyu Gong, Xingshuang Zhang, Yong Li, Meng Gao, Dongwei Li, and Guanchen Xu. 2022. "Surface Charge Transfer Doping of MoS2 Monolayer by Molecules with Aggregation-Induced Emission Effect" Nanomaterials 12, no. 1: 164. https://doi.org/10.3390/nano12010164
APA StyleSun, R., Sun, S., Liang, X., Gong, H., Zhang, X., Li, Y., Gao, M., Li, D., & Xu, G. (2022). Surface Charge Transfer Doping of MoS2 Monolayer by Molecules with Aggregation-Induced Emission Effect. Nanomaterials, 12(1), 164. https://doi.org/10.3390/nano12010164