Nanohollow Titanium Oxide Structures on Ti/FTO Glass Formed by Step-Bias Anodic Oxidation for Photoelectrochemical Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Fabrication
2.2. Photoelectrochemical Response
2.3. Material Characterization
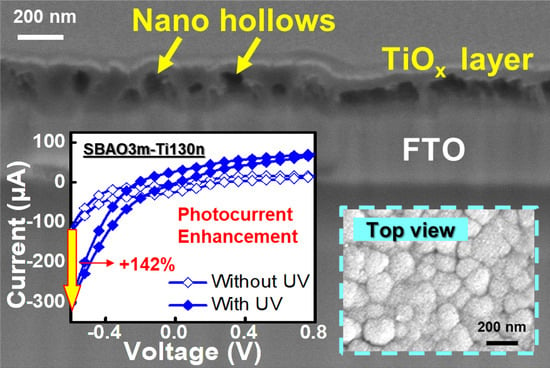
3. Results
3.1. Material Characterization
3.2. Photoelectrochemical Measurements
3.2.1. Effect of Ti Thickness
3.2.2. Effects of Anodic Oxidation Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orozco-Messana, J.; Daly, R.; Zanchetta-Chittka, I.F. Cu2O–ZnO heterojunction solar cell coupled to a Ni(OH)2-rGO-PPy supercapacitor within a porous stoneware tile. Ceram. Int. 2020, 46, 24831–24837. [Google Scholar] [CrossRef]
- Cho, S.I.; Sung, H.K.; Lee, S.J.; Kim, W.H.; Kim, D.-H.; Han, Y.S. Photovoltaic Performance of Dye-Sensitized Solar Cells Containing ZnO Microrods. Nanomaterials 2019, 9, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Wei, L.; Wei, G.; Li, Q.; Wang, D.; Chen, Y.; Yan, S.; Liu, G.; Mei, L.; Jiao, J. A self-powered UV photodetector based on TiO2 nanorod arrays. Nanoscale Res. Lett. 2013, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.J.; Hung, F.Y.; Chang, S.J.; Young, S.J. Optoelectronic characteristics of UV photodetector based on ZnO nanowire thin films. J. Alloy. Compd. 2009, 479, 674–677. [Google Scholar] [CrossRef]
- Tang, M.; Lu, S.; He, L.; Zhu, X.; Feng, W.; Zhang, W. Preparation, Characterization of ZnTiO3/ZnO Composite Materials and Their Photocatalytic Performance. Nanomaterials 2022, 12, 1345. [Google Scholar] [CrossRef]
- Tian, Q.; Wu, W.; Sun, L.; Yang, S.; Lei, M.; Zhou, J.; Liu, Y.; Xiao, X.; Ren, F.; Jiang, C.; et al. Tube-Like Ternary α-Fe2O3@SnO2@Cu2O Sandwich Heterostructures: Synthesis and Enhanced Photocatalytic Properties. ACS Appl. Mater. Interfaces 2014, 6, 13088–13097. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Zheng, L.; Hou, J.; Zheng, H.; Sun, S.; Wang, L. Machine Learning Guided Dopant Selection for Metal Oxide-Based Photoelectrochemical Water Splitting: The Case Study of Fe2O3 and CuO. Adv. Mater. 2022, 34, 2106776. [Google Scholar] [CrossRef]
- Kim, J.; Heo, J.N.; Do, J.Y.; Chava, R.K.; Kang, M. Electrochemical Synergies of Heterostructured Fe2O3-MnO Catalyst for Oxygen Evolution Reaction in Alkaline Water Splitting. Nanomaterials 2019, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Movsesyan, L.; Maijenburg, A.W.; Goethals, N.; Sigle, W.; Spende, A.; Yang, F.; Kaiser, B.; Jaegermann, W.; Park, S.-Y.; Mul, G.; et al. ZnO Nanowire Networks as Photoanode Model Systems for Photoelectrochemical Applications. Nanomaterials 2018, 8, 693. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; Mu, C.; Guo, X.; Yang, R.; Jonathan, R.; Jiao, W.; Wu, X.; Jian, X. Efficient defect engineering and in-situ carbon doping in ultra-fine TiO2 with enhanced visible-light-response photocatalytic performance. J. Alloy. Compd. 2022, 901, 163490. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water-splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, Z.; Gong, C.; Xiao, W.; Sun, L.; Lin, C. Fe3+-Doped TiO2 Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity. Nanomaterials 2016, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, W.; Yan, B.; Wu, B.; Ma, J.; Wang, X.; Qiao, B.; Tu, J.; Pei, H.; Chen, D.; et al. Gold and Platinum Nanoparticle-Functionalized TiO2 Nanotubes for Photoelectrochemical Glucose Sensing. ACS Omega 2022, 7, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; He, Z.; Zhou, C.; Yan, G.; Wu, L. MOF-Derived Porous Fe2O3 Nanoparticles Coupled with CdS Quantum Dots for Degradation of Bisphenol A under Visible Light Irradiation. Nanomaterials 2020, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Wang, L.; Shen, W.; Chen, H. Novel α-Fe2O3/CdS cornlike nanorods with enhanced photocatalytic performance. ACS Appl. Mater. Interfaces 2012, 4, 4800–4806. [Google Scholar] [CrossRef]
- Gong, A.; Feng, Y.; Liu, C.; Chen, J.; Wang, Z.; Shen, T. A promising ZnO/Graphene van der Waals heterojunction as solar cell devices: A first-principles study. Energy Rep. 2022, 8, 904–910. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, M.; Zhang, H.; Zheng, Z.; Wang, Z.; Cheng, H.; Wang, P.; Liu, Y.; Huang, B. Fabrication of ZnO Ceramics with Defects by Spark Plasma Sintering Method and Investigations of Their Photoelectrochemical Properties. Nanomaterials 2021, 11, 2506. [Google Scholar] [CrossRef]
- Chen, J.; Dai, S.; Liu, L.; Maitz, M.F.; Liao, Y.; Cui, J.; Zhao, A.; Yang, P.; Huang, N.; Wang, Y. Photo-functionalized TiO2 nanotubes decorated with multifunctional Ag nanoparticles for enhanced vascular biocompatibility. Bioact. Mater. 2021, 6, 45–54. [Google Scholar] [CrossRef]
- Konatu, R.T.; Domingues, D.D.; Escada, A.L.A.; Chaves, J.A.M.; Netipanyj, M.F.D.; Nakazato, R.Z.; Popat, K.C.; Grandini, C.R.; Alves Claro, A.P.R. Synthesis and characterization of self-organized TiO2 nanotubes grown on Ti-15Zr alloy surface to enhance cell response. Surf. Interfaces 2021, 26, 101439. [Google Scholar] [CrossRef]
- Kmentova, H.; Kment, S.; Wang, L.; Pausova, S.; Vaclavu, T.; Kuzel, R.; Han, H.; Hubicka, Z.; Zlamal, M.; Olejnicek, J.; et al. Photoelectrochemical and structural properties of TiO2 nanotubes and nanorods grown on FTO substrate: Comparative study between electrochemical anodization and hydrothermal method used for the nanostructures fabrication. Catal. Today 2017, 287, 130–136. [Google Scholar] [CrossRef]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Rho, W.Y.; Chun, M.H.; Kim, H.S.; Kim, H.M.; Suh, J.S.; Jun, B.H. Ag Nanoparticle–Functionalized Open-Ended Freestanding TiO2 Nanotube Arrays with a Scattering Layer for Improved Energy Conversion Efficiency in Dye-Sensitized Solar Cells. Nanomaterials 2016, 6, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, D.; Brillet, J.; Chen, P.; Takata, M.; Uchida, S.; Miura, H.; Sumioka, K.; Zakeeruddin, S.M.; Grätzel, M. Application of highly ordered TiO2 nanotube arrays in flexible dye-sensitized solar cells. ACS Nano 2008, 2, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, G.; Chen, X.; Wang, K. Fast charge separation and photocurrent enhancement on black TiO2 nanotubes co-sensitized with Au nanoparticles and PbS quantum dots. Electrochim. Acta 2018, 277, 244–254. [Google Scholar] [CrossRef]
- Krysa, J.; Lee, K.; Pausova, S.; Kment, S.; Hubicka, Z.; Ctvrtlik, R.; Schmuki, P. Self-organized transparent 1D TiO2 nanotubular photoelectrodes grown by anodization of sputtered and evaporated ti layers: A comparative photoelectrochemical study. Chem. Eng. J. 2017, 308, 745–753. [Google Scholar] [CrossRef]
- Yue, Z.; Lisdat, F.; Parak, W.J.; Hickey, S.G.; Tu, L.; Sabir, N.; Dorfs, D.; Bigall, N.C. Quantum-dot-based photoelectrochemical sensors for chemical and biological detection. ACS Appl. Mater. Interfaces 2013, 5, 2800–2814. [Google Scholar] [CrossRef]
- Wang, G.L.; Xu, J.J.; Chen, H.Y.; Fu, S.Z. Label-free photoelectrochemical immunoassay for α-fetoprotein detection based on TiO2/CdS hybrid. Biosens. Bioelectron. 2009, 25, 791–796. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Yang, Z.; Xu, Q.; Hu, X. A novel photoelectrochemical sensor for the organophosphorus pesticide dichlofenthion based on nanometer-sized titania coupled with a screen-printed electrode. Anal. Chem. 2011, 83, 5290–5295. [Google Scholar] [CrossRef]
- Lu, Y.J.; Purwidyantri, A.; Liu, H.L.; Wang, L.W.; Shih, C.Y.; Pijanowska, D.G.; Yang, C.M. Photoelectrochemical Detection of β-amyloid Peptides by a TiO2 Nanobrush Biosensor. IEEE Sens. J. 2020, 20, 6248–6255. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Varghese, O.K.; Paulose, M.; Mor, G.K.; A Grimes, C. Synthesis and photoelectrochemical properties of nanoporous iron (III) oxide by potentiostatic anodization. Nanotechnology 2006, 17, 4285–4291. [Google Scholar] [CrossRef]
- Zelny, M.; Kment, S.; Ctvrtlik, R.; Pausova, S.; Kmentova, H.; Tomastik, J.; Hubicka, Z.; Rambabu, Y.; Krysa, J.; Naldoni, A.; et al. TiO2 Nanotubes on Transparent Substrates: Control of Film Microstructure and Photoelectrochemical Water Splitting Performance. Catalysts 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; Ghicov, A.; Nah, Y.C.; Schmuki, P. Transparent TiO2 nanotube electrodes via thin layer anodization: Fabrication and use in electrochromic devices. Langmuir 2009, 25, 4841–4844. [Google Scholar] [CrossRef] [PubMed]
- Krýsa, J.; Krýsová, H.; Hubička, Z.; Kment, Š.; Maixner, J.; Kavan, L. Transparent rutile TiO2 films prepared by thermal oxidation of sputtered Ti on FTO glass. Photochem. Photobiol. Sci. 2019, 18, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Sahrin, N.T.; Nawaz, R.; Fai Kait, C.; Lee, S.L.; Wirzal, M.D.H. Visible Light Photodegradation of Formaldehyde over TiO2 Nanotubes Synthesized via Electrochemical Anodization of Titanium Foil. Nanomaterials 2020, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Valeeva, A.A.; Dorosheva, I.B.; Kozlova, E.A.; Sushnikova, A.A.; Kurenkova, A.Y.; Saraev, A.A.; Schroettner, H.; Rempel, A.A. Solar photocatalysts based on titanium dioxide nanotubes for hydrogen evolution from aqueous solutions of ethanol. Int. J. Hydrog. Energy 2021, 46, 16917–16924. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Cao, T.M.; Balayeva, N.O.; Pham, V.V. Thermal Treatment of Polyvinyl Alcohol for Coupling MoS2 and TiO2 Nanotube Arrays toward Enhancing Photoelectrochemical Water Splitting Performance. Catalysts 2021, 11, 857. [Google Scholar] [CrossRef]
- Lei, B.-X.; Liao, J.-Y.; Zhang, R.; Wang, J.; Su, C.-Y.; Kuang, D.-B. Ordered crystalline TiO2 nanotube arrays on transparent FTO glass for efficient dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 15228–15233. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, I.S.; Ho, K.I.; Lin, Y.T.; Chou, C.; Chan, C.F.; Lai, C.S. High polarization and low-repulsion HfO2 thin film for alkali metal ion detections by plasma system with a complementary filter. IEEE Sens. J. 2013, 13, 2459–2465. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef]
- Lin, T.; Liao, M.; Zhao, S.; Fan, H.; Zhu, X. Anodic TiO2 nanotubes produced under atmospheric pressure and in vacuum conditions. Ceram. Int. 2018, 44, 1764–1770. [Google Scholar] [CrossRef]
- Kathirvel, S.; Su, C.; Yang, C.-Y.; Shiao, Y.-J.; Chen, B.-R.; Li, W.-R. The growth of TiO2 nanotubes from sputter-deposited Ti film on transparent conducting glass for photovoltaic applications. Vacuum 2015, 118, 17–25. [Google Scholar] [CrossRef]
- Xie, W.; Li, R.; Xu, Q. Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Sci. Rep. 2018, 8, 8752. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Giurlani, W.; Sergi, L.; Crestini, E.; Calisi, N.; Poli, F.; Soavi, F.; Innocenti, M. Electrochemical stability of steel, Ti, and Cu current collectors in water-in-salt electrolyte for green batteries and supercapacitors. J. Solid State Electrochem. 2022, 26, 85–95. [Google Scholar] [CrossRef]
- Sopha, H.; Krbal, M.; Ng, S.; Prikryl, J.; Zazpe, R.; Yam, F.K.; Macak, J.M. Highly efficient photoelectrochemical and photocatalytic anodic TiO2 nanotube layers with additional TiO2 coating. Appl. Mater. Today 2017, 9, 104–110. [Google Scholar] [CrossRef]
- Han, H.; Moon, S.I.; Choi, S.; Enkhtuvshin, E.; Kim, S.J.; Jung, S.Y.; Thao, N.T.T.; Song, T. Enhanced photoelectrochemical characteristic of TiO2 nanotubes via surface plasma treatment. Ceram. Int. 2021, 47, 30741–30746. [Google Scholar] [CrossRef]
- In, S.I.; Almtoft, K.P.; Lee, H.S.; Andersen, I.H.; Qin, D.; Bao, N.; Grimes, C.A. Low temperature synthesis of transparent, vertically aligned anatase TiO2 nanowire arrays: Application to dye sensitized solar cells. Bull. Korean Chem. Soc. 2012, 33, 1989–1992. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Liu, F.; Liu, J. Effect of vanadium redox species on photoelectrochemical behavior of TiO2 and TiO2/WO3 photo-electrodes. J. Power Sources 2012, 213, 78–82. [Google Scholar] [CrossRef]
- Hao, C.; Wang, W.; Zhang, R.; Zou, B.; Shi, H. Enhanced photoelectrochemical water splitting with TiO2@Ag2O nanowire arrays via p-n heterojunction formation. Sol. Energy Mater. Sol. Cells 2018, 174, 132–139. [Google Scholar] [CrossRef]
- Ho, H.C.; Chen, K.; Nagao, T.; Hsueh, C.H. Photocurrent Enhancements of TiO2-Based Nanocomposites with Gold Nanostructures/Reduced Graphene Oxide on Nanobranched Substrate. J. Phys. Chem. C 2019, 123, 21103–21113. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Lu, Y.-J.; Pan, Y.-C.; Liu, H.-L.; Chang, J.-Y.; Sie, J.-L.; Pijanowska, D.G.; Yang, C.-M. Nanohollow Titanium Oxide Structures on Ti/FTO Glass Formed by Step-Bias Anodic Oxidation for Photoelectrochemical Enhancement. Nanomaterials 2022, 12, 1925. https://doi.org/10.3390/nano12111925
Huang C-H, Lu Y-J, Pan Y-C, Liu H-L, Chang J-Y, Sie J-L, Pijanowska DG, Yang C-M. Nanohollow Titanium Oxide Structures on Ti/FTO Glass Formed by Step-Bias Anodic Oxidation for Photoelectrochemical Enhancement. Nanomaterials. 2022; 12(11):1925. https://doi.org/10.3390/nano12111925
Chicago/Turabian StyleHuang, Chi-Hsien, Yu-Jen Lu, Yong-Chen Pan, Hui-Ling Liu, Jia-Yuan Chang, Jhao-Liang Sie, Dorota G. Pijanowska, and Chia-Ming Yang. 2022. "Nanohollow Titanium Oxide Structures on Ti/FTO Glass Formed by Step-Bias Anodic Oxidation for Photoelectrochemical Enhancement" Nanomaterials 12, no. 11: 1925. https://doi.org/10.3390/nano12111925
APA StyleHuang, C. -H., Lu, Y. -J., Pan, Y. -C., Liu, H. -L., Chang, J. -Y., Sie, J. -L., Pijanowska, D. G., & Yang, C. -M. (2022). Nanohollow Titanium Oxide Structures on Ti/FTO Glass Formed by Step-Bias Anodic Oxidation for Photoelectrochemical Enhancement. Nanomaterials, 12(11), 1925. https://doi.org/10.3390/nano12111925