Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.2. Characterization of Photocatalysts
2.3. Photocatalytic Activity Test
3. Results and Discussion
3.1. Morphology and Structure Characterization
3.2. Photocatalytic Properties
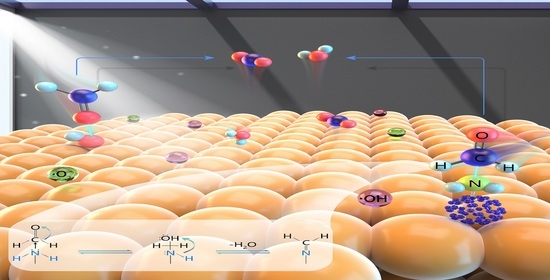
3.3. Mechanisms of Formaldehyde Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Yu, H.; Xiao, Y.; Zhang, L.; Guo, L.; Zhang, L.; Dong, X. Free-standing composite films of multiple 2D nanosheets: Synergetic photothermocatalysis/photocatalysis for efficient removal of formaldehyde under ambient condition. Chem. Eng. J. 2020, 394, 125014–125024. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huang, Y.; Cao, J.; Huang, T.; Li, R.; Zhang, Q.; Lee, S.C.; Ho, W. Exploring the photocatalytic conversion mechanism of gaseous formaldehyde degradation on TiO2-x-OV surface. J. Hazard. Mater. 2022, 424, 127217–127225. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Zhang, G.H.; Meng, Y.; Yang, C.; Ni, Z.M.; Hu, J. Kinetic and mechanistic analysis for the photodegradation of gaseous formaldehyde by core-shell CeO2@LDHs. Appl. Catal. B Environ. 2020, 278, 119266–119278. [Google Scholar] [CrossRef]
- Yuan, W.J.; Zhang, S.P.; Wu, Y.Y.; Huang, X.M.; Tian, F.H.; Liu, S.W.; Li, C.H. Enhancing the room-temperature catalytic degradation of formaldehyde through constructing surface lewis pairs on carbon-based catalyst. Appl. Catal. B Environ. 2020, 272, 118992–118999. [Google Scholar] [CrossRef]
- Zhang, C.B.; He, H. A comparative study of TiO2 supported noble metal catalysts for the oxidation of formaldehyde at room temperature. Catal. Today 2007, 126, 345–350. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, C.; Li, X.S.; Liu, J.L.; Sun, Z.G.; Shi, C.; Li, X.G.; Zhu, A.M. Photocatalytic Formaldehyde Oxidation over Plasmonic Au/TiO2 under Visible Light: Moisture Indispensability and Light Enhancement. ACS Catal. 2017, 7, 6514–6524. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, J.; Long, W.; Cui, B.; Song, K.; Dong, F.; Xu, D. Highly Efficient MnO2/AlOOH Composite Catalyst for Indoor Low-Concentration Formaldehyde Removal at Room Temperature. Inorg. Chem. 2020, 59, 7335–7343. [Google Scholar] [CrossRef]
- Robert, B.; Nallathambi, G. Indoor formaldehyde removal by catalytic oxidation, adsorption and nanofibrous membranes: A review. Environ. Chem. Lett. 2021, 19, 2551–2579. [Google Scholar] [CrossRef]
- Vipin, S.; Varun, G.; Paramvir, S.; Alok, G. Abatement of formaldehyde with photocatalytic and catalytic oxidation: A review. Int. J. Chem. React. Eng. 2020, 19, 1–29. [Google Scholar]
- Adilah, S.; Salvador, E.; Thanita, A.; Hugo, D.L.; Siriluk, C. Photocatalytic Conversion of Organic Pollutants in Air: Quantum Yields Using a Silver/Nitrogen/TiO2 Mesoporous Semiconductor under Visible Light. Catalysts 2021, 11, 529–555. [Google Scholar]
- Yan, S.; Yu, J.; Zhu, B.; Qiao, K.; Cai, X.; Yuan, X.; Li, C. Photocatalytic Application of TiO2-Loaded Viscose-Based Activated Carbon Fibers Composite Catalyst: Degradation of Low Concentration Formaldehyde and Simultaneous Anti-Microbe. ECS J. Solid State Sc. 2021, 10, 011002–011012. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.J.; Zhang, W.; Liu, Z.F.; Zeng, G.M.; Shao, B.B.; Liang, Q.H.; He, Q.Y.; Yuan, X.Z.; Huang, D.L.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579–118607. [Google Scholar] [CrossRef]
- Lian, Z.; Xu, P.; Wang, W.; Zhang, D.; Xiao, S.; Li, X.; Li, G. C60-decorated CdS/TiO2 mesoporous architectures with enhanced photostability and photocatalytic activity for H2 evolution. ACS Appl. Mater. Inter. 2015, 7, 4533–4540. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H.; Kohtani, S. Photocatalytic single electron transfer reactions on TiO2 semiconductor. Sci. China Chem. 2019, 62, 1439–1449. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, F.; Zhang, Z.; Sun, G.; Xu, H.; Chai, P.; Huang, W. Surface Chemistry of Formaldehyde on Rutile TiO2(011)-(2×1) Surface: Photocatalysis Versus Thermal-Catalysis. J. Phys. Chem. C 2017, 121, 25921–25929. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Zhang, Z.S.; Yi, H.Q.; Zeng, L.; Tang, C.; Huang, L.M.; Gu, M. Covalently bonded 2D/2D O-g-C3N4/TiO2 heterojunction for enhanced visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 237, 1130–1138. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Chai, Y.; Wu, B.; Wang, C. Surface modification of TiO2 nanosheets with fullerene and zinc-phthalocyanine for enhanced photocatalytic reduction under solar-light irradiation. Sci. China Mater. 2020, 63, 2251–2260. [Google Scholar] [CrossRef]
- Huo, P.; Shi, X.; Zhang, W.; Kumar, P.; Liu, B. An overview on the incorporation of graphene quantum dots on TiO2 for enhanced performances. J. Mater. Sci. 2021, 56, 6031–6051. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Li, X.; Cheng, B.; Liu, G.; Jaroniec, M. Enhanced performance of NaOH-modified Pt/TiO2 toward room temperature selective oxidation of formaldehyde. Environ. Sci. Technol. 2013, 47, 2777–2783. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Quiroz Torres, J.; Royer, S.; Bellat, J.P.; Giraudon, J.M.; Lamonier, J.F. Formaldehyde: Catalytic oxidation as a promising soft way of elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, G.; Cheng, B. Enhanced photocatalytic activity of bimodal mesoporous titania powders by C60 modification. Dalton Trans. 2011, 40, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, C.; Dai, K.; Cai, P.; Chen, H.; Yang, C.; Huang, Q. Fullerene C70-TiO2 hybrids with enhanced photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2015, 3, 21090–21098. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly(l-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C 2016, 60, 184–194. [Google Scholar] [CrossRef]
- Meng, Z.D.; Peng, M.M.; Zhu, L.; Oh, W.C.; Zhang, F.J. Fullerene modification CdS/TiO2 to enhancement surface area and modification of photocatalytic activity under visible light. Appl. Catal. B Environ. 2012, 113, 141–149. [Google Scholar] [CrossRef]
- Li, Q.; Xu, L.; Luo, K.-W.; Huang, W.-Q.; Wang, L.-L.; Li, X.-F.; Huang, G.-F.; Yu, Y.-B. Insights into enhanced visible-light photocatalytic activity of C60 modified g-C3N4 hybrids: The role of nitrogen. Phys. Chem. Chem. Phys. 2016, 18, 33094–33102. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, L.; Wang, Y.J.; Yao, W.Q.; Zhu, Y.F. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152, 262–270. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials 2020, 13, 2924. [Google Scholar] [CrossRef]
- Bai, W.; Krishna, V.; Wang, J.; Moudgil, B.; Koopman, B. Enhancement of nano titanium dioxide photocatalysis in transparent coatings by polyhydroxy fullerene. Appl. Catal. B Environ. 2012, 125, 128–135. [Google Scholar] [CrossRef]
- Li, Q.; Hong, L.; Li, H.; Liu, C. Graphene oxide-fullerene C60 (GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens. Bioelectron. 2017, 89, 477–482. [Google Scholar] [CrossRef]
- Meng, Z.D.; Zhu, L.; Ye, S.; Sun, Q.; Ullah, K.; Cho, K.Y.; Oh, W.C. Fullerene modification CdSe/TiO2 and modification of photocatalytic activity under visible light. Nanoscale Res. Lett. 2013, 8, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Chen, Z.-C.; Tian, H.-R.; Xu, Y.-Y.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Implications of Nitrogen Doping on Geometrical and Electronic Structure of the Fullerene Dimers. Chin. J. Chem. 2021, 39, 93–98. [Google Scholar] [CrossRef]
- Na, C.J.; Yoo, M.J.; Tsang, D.C.W.; Kim, H.W.; Kim, K.H. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef]
- Wu, K.; Kong, X.Y.; Xiao, K.; Wei, Y.; Zhu, C.C.; Zhou, R.; Si, M.T.; Wang, J.J.; Zhang, Y.Q.; Wen, L.P. Engineered Smart Gating Nanochannels for High Performance in Formaldehyde Detection and Removal. Adv. Funct. Mater. 2019, 29, 1807953. [Google Scholar] [CrossRef]
- Wu, L.; Qin, Z.; Zhang, L.; Meng, T.; Yu, F.; Ma, J. CNT-enhanced amino-functionalized graphene aerogel adsorbent for highly efficient removal of formaldehyde. New J. Chem. 2017, 41, 2527–2533. [Google Scholar] [CrossRef]
- Vikrant, K.; Cho, M.; Khan, A.; Kim, K.H.; Ahn, W.S.; Kwon, E.E. Adsorption properties of advanced functional materials against gaseous formaldehyde. Environ. Res. 2019, 178, 108672–108686. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, X.; Huang, Y.; Xu, S.; Su, X.; Pan, X.; Xu, J.; Wang, A.; Liang, C.; Wang, X.; et al. A Schiff base modified gold catalyst for green and efficient H2 production from formic acid. Energy Environ. Sci. 2015, 8, 3204–3207. [Google Scholar] [CrossRef]
- Carter, E.M.; Katz, L.E.; Speitel, G.E., Jr.; Ramirez, D. Gas-phase formaldehyde adsorption isotherm studies on activated carbon: Correlations of adsorption capacity to surface functional group density. Environ. Sci. Technol. 2011, 45, 6498–6503. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.W.; Wang, T.S.; Nie, M.Z.; Li, J.J.; Wei, Z.X.; Jiang, L.; Wang, C.R. Ethylenediamine functionalized fullerene nanoparticles as independent electron transport layers for high-efficiency inverted polymer solar cells. J. Mater. Chem. A 2017, 5, 947–951. [Google Scholar] [CrossRef]
- Lamparth, I.; Hirsch, A. Water-soluble Malonic Acid Derivatives of C60 with a Defined Three-dimensional Structure. J. Chem. Soc. Chem. Commun. 1994, 14, 1727–1728. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Enhancement of photocatalytic decarboxylation on TiO2 by water-induced change in adsorption-mode. Appl. Catal. B Environ. 2018, 224, 376–382. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, H.N.; Song, W.J.; Ji, H.W.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Activation of Water in Titanium Dioxide Photocatalysis by Formation of Surface Hydrogen Bonds: An In Situ IR Spectroscopy Study. Angew. Chem. Int. Ed. 2015, 54, 5905–5909. [Google Scholar] [CrossRef]
- Qi, K.Z.; Selvaraj, R.; Al Fahdi, T.; Al-Kindy, S.; Kim, Y.; Wang, G.C.; Tai, C.W.; Sillanpaa, M. Enhanced photocatalytic activity of anatase-TiO2 nanoparticles by fullerene modification: A theoretical and experimental study. Appl. Surf. Sci. 2016, 387, 750–758. [Google Scholar] [CrossRef]
- Xiong, M.; Tao, Y.; Zhao, Z.; Zhu, Q.; Jin, X.; Zhang, S.; Chen, M.; Li, G. Porous g-C3N4/TiO2 foam photocatalytic filter for treating NO indoor gas. Environ. Sci. Nano 2021, 8, 1571–1579. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, W.-X. Heterostructured graphitic carbon nitride/titanium dioxide for enhanced photodegradation of low-concentration formaldehyde under visible light. J. Photochem. Photobiol. A 2019, 378, 66–73. [Google Scholar] [CrossRef]
- Chechia, H.; Wei-Fan, T.; Wei-Han, W.; Kun-Yi Andrew, L.; Miao-Ting, L.; Keizo, N. Hydroxylation and sodium intercalation on g-C3N4 for photocatalytic removal of gaseous formaldehyde. Carbon 2021, 175, 467–477. [Google Scholar]
- Yu, L.; Wang, L.; Sun, X.; Ye, D. Enhanced photocatalytic activity of rGO/TiO2 for the decomposition of formaldehyde under visible light irradiation. J. Environ. Sci. 2018, 73, 138–146. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, G. Fabrication of AgFeO2/g-C3N4 nanocatalyst with enhanced and stable photocatalytic performance. Appl. Surf. Sci. 2017, 391, 415–422. [Google Scholar] [CrossRef]
- Song, S.; Lu, C.; Wu, X.; Jiang, S.; Sun, C.; Le, Z. Strong base g-C3N4 with perfect structure for photocatalytically eliminating formaldehyde under visible-light irradiation. Appl. Catal. B Environ. 2018, 227, 145–152. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, W.-X. A simple method to prepare g-C3N4-TiO2/waste zeolites as visible-light-responsive photocatalytic coatings for degradation of indoor formaldehyde. J. Hazard. Mater. 2019, 368, 468–476. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, C.; Jiang, S.; Wu, X.; Song, S. Efficient evolution of reactive oxygen species over the coordinated π-delocalization g-C3N4 with favorable charge transfer for sustainable pollutant elimination. Appl. Catal. B Environ. 2019, 249, 282–291. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Li, J.; Wang, K.; Zhang, G. Z-scheme g-C3N4@CsxWO3 heterostructure as smart window coating for UV isolating, Vis penetrating, NIR shielding and full spectrum photocatalytic decomposing VOCs. Appl. Catal. B Environ. 2018, 229, 218–226. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; An, X.; Huang, J. Preparation of a novel composite comprising biochar skeleton and “chrysanthemum” g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde. Appl. Surf. Sci. 2019, 487, 1262–1270. [Google Scholar] [CrossRef]
- Lan, Z.; Yu, Y.; Yao, J.; Cao, Y. The band structure and photocatalytic mechanism of MoS2-modified C3N4 photocatalysts with improved visible photocatalytic activity. Mater. Res. Bull. 2018, 102, 433–439. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Tao, T.; Zhao, Y.; Wang, P.; Ding, Z.; Chen, M. Controlled synthesis of Bi2O3/TiO2 catalysts with mixed alcohols for the photocatalytic oxidation of HCHO. Environ. Technol. 2019, 40, 1937–1947. [Google Scholar] [CrossRef]
- Han, X.; Han, Z.; Zhao, J.; Zhao, X. Photocatalytic degradation of formaldehyde by PAN nonwoven supported Fe(III) catalysts under visible light irradiation. New J. Chem. 2017, 41, 9380–9387. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Le, Y.; Cheng, B.; Yu, J. Hierarchical honeycomb-like Pt/NiFe-LDH/rGO nanocomposite with excellent formaldehyde decomposition activity. Chem. Eng. J. 2019, 365, 378–388. [Google Scholar] [CrossRef]
- Qin, G.; Wu, X.; Zhang, H. Rational design of TiO2-V2O5-C nanostructure grafted by N-doped graphene with enhanced photocatalysis and lithium ion store performances. Rsc Adv. 2014, 4, 52438–52450. [Google Scholar] [CrossRef]
- Dou, H.L.; Long, D.; Rao, X.; Zhang, Y.P.; Qin, Y.M.; Pan, F.; Wu, K. Photocatalytic Degradation Kinetics of Gaseous Formaldehyde Flow Using TiO2 Nanowires. ACS Sustain. Chem. Eng. 2019, 7, 4456–4465. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Zheng, H.; Zhang, P. Hierarchical Z-scheme 1D/2D architecture with TiO2 nanowires decorated by MnO2 nanosheets for efficient adsorption and full spectrum photocatalytic degradation of organic pollutants. Catal. Sci. Technol. 2020, 10, 3603–3612. [Google Scholar] [CrossRef]
- Fontmorin, J.M.; Burgos Castillo, R.C.; Tang, W.Z.; Sillanpaa, M. Stability of 5,5-dimethyl-1-pyrroline-N-oxide as a spin-trap for quantification of hydroxyl radicals in processes based on Fenton reaction. Water Res. 2016, 99, 24–32. [Google Scholar] [CrossRef]
- Hu, Y.D.; Xie, X.F.; Wang, X.; Wang, Y.; Zeng, Y.; Pui, D.Y.H.; Sun, J. Visible-Light Upconversion Carbon Quantum Dots Decorated TiO2 for the Photodegradation of Flowing Gaseous Acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. [Google Scholar] [CrossRef]
- Nomura, A.; Jones, C.W. Enhanced Formaldehyde-Vapor Adsorption Capacity of Polymeric Amine-Incorporated Aminosilicas. Chem. Eur. J. 2014, 20, 6381–6390. [Google Scholar] [CrossRef]







| Species | Assignment | Wavenumber (cm−1) | Refs. |
|---|---|---|---|
| DOM/Hemiaminal | ν(CO) δ(CH2) | 1114, 1174 1415 | [6,37] |
| Formate/Carbonate | νs(COO) νas(COO) | 1359 1578 | [6,7] |
| Hydroxy/Water | ν(OH) δ(OH) | 3656, 3511, 3693 1716, 1677 | [6,55,56] |
| Hemiaminal/Imine | ν(C-N) ν(C=N) | 1251, 1300, 1360 1573 | [34,36,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Wang, T.; Wu, B.; Wang, C. Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation. Nanomaterials 2022, 12, 2366. https://doi.org/10.3390/nano12142366
Fan J, Wang T, Wu B, Wang C. Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation. Nanomaterials. 2022; 12(14):2366. https://doi.org/10.3390/nano12142366
Chicago/Turabian StyleFan, Jingbiao, Tao Wang, Bo Wu, and Chunru Wang. 2022. "Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation" Nanomaterials 12, no. 14: 2366. https://doi.org/10.3390/nano12142366
APA StyleFan, J., Wang, T., Wu, B., & Wang, C. (2022). Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation. Nanomaterials, 12(14), 2366. https://doi.org/10.3390/nano12142366