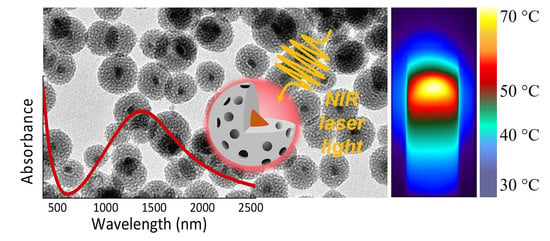
NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Colloidal Cu2−xS Nanocrystals
2.3. Synthesis of Mesoporous Silica Nanoparticles (MSN)
2.4. Synthesis of Cu2−xS NCs Coated by a Mesoporous Silica Shell
2.5. Characterization Techniques
2.6. Evaluation of Photothermal Properties
2.7. Cell Culture and Cell Proliferation Assay
3. Results
3.1. Design of the Cu2−xS NCs and Mesoporous Silica Shell
3.2. Photothermal Conversion Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The Most Effective Gold Nanorod Size for Plasmonic Photothermal Therapy: Theory and In Vitro Experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Z.; Yang, F.; Yin, Y. Plasmonic Nanostructures for Photothermal Conversion. Small Sci. 2021, 1, 2000055. [Google Scholar] [CrossRef]
- Gellini, C.; Feis, A. Optothermal properties of plasmonic inorganic nanoparticles for photoacoustic applications. Photoacoustics 2021, 23, 100281. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Yougbaré, S.; Chou, H.-L.; Yang, C.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.-R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- González-Ruíz, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Escudero-Castellanos, A.; Ocampo-García, B.; Luna-Gutiérrez, M.; Santos-Cuevas, C.; Morales-Avila, E.; Isaac-Olivé, K. In vitro and in vivo synergistic effect of radiotherapy and plasmonic photothermal therapy on the viability of cancer cells using 177Lu–Au-NLS-RGD-Aptamer nanoparticles under laser irradiation. J. Radioanal. Nucl. Chem. 2018, 318, 1913–1921. [Google Scholar] [CrossRef]
- Taylor, M.L.; Wilson, R.E.; Amrhein, K.D.; Huang, X. Gold Nanorod-Assisted Photothermal Therapy and Improvement Strategies. Bioengineering 2022, 9, 200. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Quantifying the Efficiency of Plasmonic Materials for Near-Field Enhancement and Photothermal Conversion. J. Phys. Chem. C 2015, 119, 25518–25528. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.-M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.C.; Zhang, Q.; Manthiram, K.; Ye, X.; Lomont, J.P.; Harris, C.B.; Weller, H.; Alivisatos, A.P. Study of Heat Transfer Dynamics from Gold Nanorods to the Environment via Time-Resolved Infrared Spectroscopy. ACS Nano 2016, 10, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Ding, Y.; Sun, S. Understanding the photothermal effect of gold nanostars and nanorods for biomedical applications. RSC Adv. 2014, 4, 30375–30383. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape effects of gold nanoparticles in photothermal cancer therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Jauffred, L.; Samadi, A.; Klingberg, H.; Bendix, P.M.; Oddershede, L.B. Plasmonic Heating of Nanostructures. Chem. Rev. 2019, 119, 8087–8130. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, Y.; Zhu, J. Quantifying and Comparing the Near-Field Enhancement, Photothermal Conversion, and Local Heating Performance of Plasmonic SiO2@Au Core-Shell Nanoparticles. Plasmonics 2019, 14, 1019–1027. [Google Scholar] [CrossRef]
- Hernández Montoto, A.; Montes, R.; Samadi, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Aznar, E.; Ibañez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Ghosh, S.K. Gold Nanostars in Plasmonic Photothermal Therapy: The Role of Tip Heads in the Thermoplasmonic Landscape. J. Phys. Chem. C 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef]
- González-Rubio, G.; Díaz-Núñez, P.; Rivera, A.; Prada, A.; Tardajos, G.; González-Izquierdo, J.; Bañares, L.; Llombart, P.; Macdowell, L.G.; Palafox, M.A.; et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science 2017, 358, 640–644. [Google Scholar] [CrossRef]
- Harris-Birtill, D.; Singh, M.; Zhou, Y.; Shah, A.; Ruenraroengsak, P.; Gallina, M.E.; Hanna, G.B.; Cass, A.E.G.; Porter, A.E.; Bamber, J.; et al. Gold nanorod reshaping in vitro and in vivo using a continuous wave laser. PLoS ONE 2017, 12, e0185990. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, L.; Khlebtsov, B.N.; Centi, S.; Khlebtsov, N.G.; Pini, R.; Ratto, F. Photostability of Contrast Agents for Photoacoustics: The Case of Gold Nanorods. Nanomaterials 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.-W.; Liu, G.; Ku, Y.-C.; Kuo, M.-K. Plasmon-Enhanced Photothermal and Optomechanical Deformations of a Gold Nanoparticle. Nanomaterials 2020, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kaur, S.; Kim, Y.D.; Kim, J.-M.; Lee, S.H.; Lim, D.-K. Precise control over the silica shell thickness and finding the optimal thickness for the peak heat diffusion property of AuNR@SiO2. J. Mater. Chem. B 2022, 10, 364–372. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Aglyamov, S.; Emelianov, S. Environment-Dependent Generation of Photoacoustic Waves from Plasmonic Nanoparticles. Small 2012, 8, 47–52. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Frey, W.; Kim, S.; Kruizinga, P.; Homan, K.; Emelianov, S. Silica-Coated Gold Nanorods as Photoacoustic Signal Nanoamplifiers. Nano Lett. 2011, 11, 348–354. [Google Scholar] [CrossRef]
- Lombard, J.; Biben, T.; Merabia, S. Electron–phonon effects on the photoacoustic response of gold core–silica shell nanoparticles: From the linear regime to nanocavitation. J. Chem. Phys. 2022, 156, 084701. [Google Scholar] [CrossRef]
- Alkurdi, A.; Lombard, J.; Detcheverry, F.; Merabia, S. Enhanced Heat Transfer with Metal-Dielectric Core-Shell Nanoparticles. Phys. Rev. Appl. 2020, 13, 034036. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, M.; Wang, P. Recent Advances in Small Copper Sulfide Nanoparticles for Molecular Imaging and Tumor Therapy. Mol. Pharm. 2019, 16, 3322–3332. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.-Q.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Recent Advances in Combination of Copper Chalcogenide-Based Photothermal and Reactive Oxygen Species-Related Therapies. ACS Biomater. Sci. Eng. 2020, 6, 4799–4815. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Skripka, A.; Besteiro, L.V.; Benayas, A.; Wang, Z.; Govorov, A.O.; Canton, P.; Vetrone, F. Highly Efficient Copper Sulfide-Based Near-Infrared Photothermal Agents: Exploring the Limits of Macroscopic Heat Conversion. Small 2018, 14, 1803282. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window. Nano-Micro Lett. 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, T.; Han, Y.; Zou, L.; Yang, H.; Jiang, J.; Liu, S.; Zhao, Q.; Huang, W. In Situ Growth of CuS/SiO2-Based Multifunctional Nanotherapeutic Agents for Combined Photodynamic/Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 31008–31018. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Goel, S.; Graves, S.A.; Orbay, H.; Ehlerding, E.B.; Shi, S.; Theuer, C.P.; Nickles, R.J.; Cai, W. In Vivo Tumor Vasculature Targeting of CuS@MSN Based Theranostic Nanomedicine. ACS Nano 2015, 9, 3926–3934. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Chen, S.; Wang, R.; Chen, Q.; Li, J.; Luo, Y.; Wang, X.; Chen, H. Photothermal Fenton Nanocatalysts for Synergetic Cancer Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2020, 12, 30145–30154. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, Y.; Yu, Y.; Chen, X.; Wei, X.; Zhang, X.; Li, C. Single Continuous Near-Infrared Laser-Triggered Photodynamic and Photothermal Ablation of Antibiotic-Resistant Bacteria Using Effective Targeted Copper Sulfide Nanoclusters. ACS Appl. Mater. Interfaces 2017, 9, 30470–30479. [Google Scholar] [CrossRef]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic Copper Sulfide Nanocrystals Exhibiting Near-Infrared Photothermal and Photodynamic Therapeutic Effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Du, Y.; Ren, J.; Qu, X. Using Plasmonic Copper Sulfide Nanocrystals as Smart Light-Driven Sterilants. ACS Nano 2015, 9, 10335–10346. [Google Scholar] [CrossRef]
- Hou, S.; Mahadevegowda, S.H.; Mai, V.C.; Chan-Park, M.B.; Duan, H. Glycosylated Copper Sulfide Nanocrystals for Targeted Photokilling of Bacteria in the Near-Infrared II Window. Adv. Ther. 2019, 2, 1900052. [Google Scholar] [CrossRef]
- Davidson, M.; Ji, Y.; Leong, G.J.; Kovach, N.C.; Trewyn, B.G.; Richards, R.M. Hybrid Mesoporous Silica/Noble-Metal Nanoparticle Materials—Synthesis and Catalytic Applications. ACS Appl. Nano Mater. 2018, 1, 4386–4400. [Google Scholar] [CrossRef]
- He, Y.; Shen, Y.; Zhou, S.; Wu, Y.; Yuan, Z.; Wei, C.; Gui, L.; Chen, Y.; Gu, Y.; Chen, H. Near infrared dye loaded copper sulfide-apoferritin for tumor imaging and photothermal therapy. RSC Adv. 2018, 8, 14268–14279. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, Z.; Chi, J.; Sun, Y.; Sun, Y. Recent progress in synergistic chemotherapy and phototherapy by targeted drug delivery systems for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 817–830. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, R.; Chen, L.; Nedilko, A.; Sánchez-Iglesias, A.; Rix, A.; Lederle, W.; Pathak, V.; Lammers, T.; von Plessen, G.; Kostarelos, K.; et al. Optimizing the Geometry of Photoacoustically Active Gold Nanoparticles for Biomedical Imaging. ACS Photonics 2020, 7, 646–652. [Google Scholar] [CrossRef]
- Han, S.; Bouchard, R.; Sokolov, K.V. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed. Opt. Express 2019, 10, 3472–3483. [Google Scholar] [CrossRef] [PubMed]
- Giancaspro, M.; Sibillano, T.; Panzarea, F.; Giannini, C.; Schmitzer, S.; Vischio, F.; Depalo, N.; Agostiano, A.; Curri, M.L.; Striccoli, M.; et al. Cu2−xS nanocrystal synthesis: A chemical toolbox for controlling nanocrystal geometry, phase and plasmonic behavior. Mater. Chem. Front. 2021, 5, 1341–1354. [Google Scholar] [CrossRef]
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High Surface Area Mesoporous Silica Nanoparticles with Tunable Size in the Sub-Micrometer Regime: Insights on the Size and Porosity Control Mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesth. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Hu, X.; Zrazhevskiy, P.; Gao, X. Encapsulation of Single Quantum Dots with Mesoporous Silica. Ann. Biomed. Eng. 2009, 37, 1960–1966. [Google Scholar] [CrossRef]
- Suteewong, T.; Sai, H.; Lee, J.; Bradbury, M.; Hyeon, T.; Gruner, S.M.; Wiesner, U. Ordered mesoporous silica nanoparticles with and without embedded iron oxide nanoparticles: Structure evolution during synthesis. J. Mater. Chem. 2010, 20, 7807–7814. [Google Scholar] [CrossRef]
- Cichos, J.; Karbowiak, M. A general and versatile procedure for coating of hydrophobic nanocrystals with a thin silica layer enabling facile biofunctionalization and dye incorporation. J. Mater. Chem. B 2014, 2, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lei, C.; Wang, Y.; Yu, C. Dendritic Mesoporous Nanoparticles: Structure, Synthesis and Properties. Angew. Chem. Int. Ed. 2022, 61, e202112752. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Long, M.; Liang, S.; Xu, H. Tuning pore size of mesoporous silica nanoparticles simply by varying reaction parameters. J. Non-Cryst. Solids 2017, 457, 9–12. [Google Scholar] [CrossRef]
- Vischio, F.; Fanizza, E.; De Bellis, V.; Sibillano, T.; Ingrosso, C.; Giannini, C.; Laquintana, V.; Denora, N.; Agostiano, A.; Striccoli, M.; et al. Near-Infrared Absorbing Solid Lipid Nanoparticles Encapsulating Plasmonic Copper Sulfide Nanocrystals. J. Phys. Chem. C 2019, 123, 23205–23213. [Google Scholar] [CrossRef]
- Rinoldi, C.; Zargarian, S.S.; Nakielski, P.; Li, X.; Liguori, A.; Petronella, F.; Presutti, D.; Wang, Q.; Costantini, M.; De Sio, L.; et al. Nanotechnology-Assisted RNA Delivery: From Nucleic Acid Therapeutics to COVID-19 Vaccines. Small Methods 2021, 5, 2100402. [Google Scholar] [CrossRef]
- De Angelis, B.; Depalo, N.; Petronella, F.; Quintarelli, C.; Curri, M.L.; Pani, R.; Calogero, A.; Locatelli, F.; De Sio, L. Stimuli-responsive nanoparticle-assisted immunotherapy: A new weapon against solid tumours. J. Mater. Chem. B 2020, 8, 1823–1840. [Google Scholar] [CrossRef]
- Knights, O.; Freear, S.; McLaughlan, J.R. Improving Plasmonic Photothermal Therapy of Lung Cancer Cells with Anti-EGFR Targeted Gold Nanorods. Nanomaterials 2020, 10, 1307. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Khosravi Khorashad, L.; Besteiro, L.V.; Wang, Z.; Valentine, J.; Govorov, A.O. Localization of Excess Temperature Using Plasmonic Hot Spots in Metal Nanostructures: Combining Nano-Optical Antennas with the Fano Effect. J. Phys. Chem. C 2016, 120, 13215–13226. [Google Scholar] [CrossRef]
- Nicolás-Boluda, A.; Vaquero, J.; Laurent, G.; Renault, G.; Bazzi, R.; Donnadieu, E.; Roux, S.; Fouassier, L.; Gazeau, F. Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano 2020, 14, 5738–5753. [Google Scholar] [CrossRef] [PubMed]







| Sample Name | CTAB (mM) | Cu2−xS (μM) | TEOS (mL) |
|---|---|---|---|
| NPL@MSS_05 | 5 | 0.2 | 0.5 |
| NPL@MSS_03 | 5 | 0.2 | 0.3 |
| NS@MSS_05 | 5 | 0.2 | 0.5 |
| NS@MSS_03 | 5 | 0.2 | 0.3 |
| Sample Name | Size by DLS (nm) | PDI | ζ Potential Value (mV) |
|---|---|---|---|
| MSN | 51 | 0.38 ± 0.05 | −31.2 ± 6.8 |
| NPL@MSS_05 | 93 | 0.30 ± 0.02 | −27.3 ± 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanizza, E.; Mastrogiacomo, R.; Pugliese, O.; Guglielmelli, A.; De Sio, L.; Castaldo, R.; Scavo, M.P.; Giancaspro, M.; Rizzi, F.; Gentile, G.; et al. NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials 2022, 12, 2545. https://doi.org/10.3390/nano12152545
Fanizza E, Mastrogiacomo R, Pugliese O, Guglielmelli A, De Sio L, Castaldo R, Scavo MP, Giancaspro M, Rizzi F, Gentile G, et al. NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials. 2022; 12(15):2545. https://doi.org/10.3390/nano12152545
Chicago/Turabian StyleFanizza, Elisabetta, Rita Mastrogiacomo, Orietta Pugliese, Alexa Guglielmelli, Luciano De Sio, Rachele Castaldo, Maria Principia Scavo, Mariangela Giancaspro, Federica Rizzi, Gennaro Gentile, and et al. 2022. "NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion" Nanomaterials 12, no. 15: 2545. https://doi.org/10.3390/nano12152545
APA StyleFanizza, E., Mastrogiacomo, R., Pugliese, O., Guglielmelli, A., De Sio, L., Castaldo, R., Scavo, M. P., Giancaspro, M., Rizzi, F., Gentile, G., Vischio, F., Carrieri, L., De Pasquale, I., Mandriota, G., Petronella, F., Ingrosso, C., Lavorgna, M., Comparelli, R., Striccoli, M., ... Depalo, N. (2022). NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials, 12(15), 2545. https://doi.org/10.3390/nano12152545