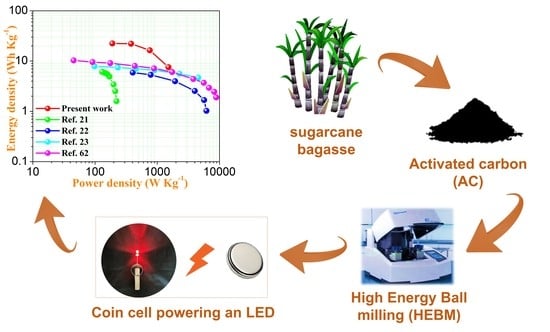
Enhanced Electrochemical Performance of Sugarcane Bagasse-Derived Activated Carbon via a High-Energy Ball Milling Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
2.3.1. Electrochemical Performance in a Three-Electrode System with 3 M KOH
2.3.2. Electrochemical Performance in a Two-Electrode System with a 1 M LiPF6 Electrolyte
3. Results and Discussion
3.1. XRD and Raman Spectroscopy
3.2. FE-SEM and Zeta Particle Size Analysis
3.3. N2 Adsorption–Desorption
3.4. XPS and FT–IR
3.5. Electrochemical Properties
| Material | Specific Surface Area (m2 g−1) | Specific Capacitance (F g−1) | Scan Rate/Current Density | Electrolytes | Cell (3E/2E) | Ref. |
|---|---|---|---|---|---|---|
| Bamboo | 1472 | 146 | 0.2 A g−1 | EMIM TFSI | 2E | [52] |
| Corns stalks | 540 | 213 | 1 A g−1 | 6 M KOH | 3E | [53] |
| Wastepaper | 416 | 180 | 2 mV s−1 | 6 M KOH | 3E | [54] |
| Banana fibers | 1097 | 74 | 0.5 A g−1 | 1 M Na2SO4 | 3E | [55] |
| Coconut shells | 1874 | 268 | 1 A g−1 | 6 M KOH | 3E | [56] |
| Rice husks | 2696 | 112 | 1 A g−1 | 1 M Na2SO4 | 3E | [57] |
| Corn stalk core | 2350 | 140 | 1 A g−1 | 3 M KOH | 3E | [10] |
| Corn silk | 2285 | 160 | 1 A g−1 | 6 M KOH | 2E | [58] |
| Rice straw | 396 | 112 | 1 A g−1 | 1 M H2SO4 | 3E | [57] |
| Pistachio shells | 1009 | 125 | 10 mV s−1 | 1 M HNO3 | 3E | [59] |
| Beer lees | 3560 | 188 | 1 mA cm−2 | 0.1 M H2SO4 | 3E | [60] |
| Natural wood | 2925 | 200 | 2 mV s−1 | 6 M KOH | 3E | [61] |
| Onion peels | - | 127 | 0.75 A g−1 | 1 M H2SO4 | 2E | [62] |
| Sugarcane bagasse | 684 | 216 | 0.2 A g−1 | 2 M KOH | 3E | [22] |
| Sugarcane bagasse | 1437 | 186 | 0.5 A g−1 | 6 M KOH | 2E | [23] |
| Sugarcane bagasse | 1207 | 228 | 0.2 A g−1 | 6 M KOH | 2E | [24] |
| Sugarcane bagasse | 1150–2632 | 257 | 0.5 A g−1 | 3 M KOH | 3E | This work |
| 110 | 0.25 A g−1 | 1 M LiPF6 | 2E | This work |
3.6. Coin Cell Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.M.M.; Imae, T. Chapter 10—Graphene-Based Nanolayers Toward Energy Storage Device. In Nanolayer Research; Imae, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–389. [Google Scholar]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Zheng, W. A Review for Aqueous Electrochemical Supercapacitors. Front. Energy Res. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Vix-Guterl, C.; Saadallah, S.; Jurewicz, K.; Frackowiak, E.; Reda, M.; Parmentier, J.; Patarin, J.; Beguin, F. Supercapacitor electrodes from new ordered porous carbon materials obtained by a templating procedure. Mater. Sci. Eng. B 2004, 108, 148–155. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Laheäär, A.; Kurig, H.; Jänes, A.; Lust, E. LiPF6 based ethylene carbonate–dimethyl carbonate electrolyte for high power density electrical double layer capacitor. Electrochim. Acta 2009, 54, 4587–4594. [Google Scholar] [CrossRef]
- Azam, M.A.; Jantan, N.H.; Dorah, N.; Seman, R.N.A.R.; Manaf, N.S.A.; Kudin, T.I.T.; Yahya, M.Z.A. Activated carbon and single-walled carbon nanotube based electrochemical capacitor in 1 M LiPF6 electrolyte. Mater. Res. Bull. 2015, 69, 20–23. [Google Scholar] [CrossRef]
- Liu, H.; Song, H.; Chen, X.; Zhang, S.; Zhou, J.; Ma, Z. Effects of nitrogen- and oxygen-containing functional groups of activated carbon nanotubes on the electrochemical performance in supercapacitors. J. Power Sources 2015, 285, 303–309. [Google Scholar] [CrossRef]
- Yu, K.; Zhu, H.; Qi, H.; Liang, C. High surface area carbon materials derived from corn stalk core as electrode for supercapacitor. Diam. Relat. Mater. 2018, 88, 18–22. [Google Scholar] [CrossRef]
- Volperts, A.; Dobele, G.; Zhurinsh, A.; Vervikishko, D.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitor electrodes with a sulfuric acid electrolyte. New Carbon Mater. 2017, 32, 319–326. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.-P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Kishore Babu, S.; Maiyalagan, T.; Rajadurai, N.; Vijayakumar, T. Activated carbon derived from bamboo-leaf with effect of various aqueous electrolytes as electrode material for supercapacitor applications. Mater. Lett. 2021, 301, 130335. [Google Scholar] [CrossRef]
- Haghighi Poudeh, L.; Berktas, I.; Ali, H.Q.; Saner Okan, B.; Yıldız, M. Toward Next-Generation Carbon-Based Materials Derived from Waste and Biomass for High-Performance Energy Applications. Energy Technol. 2020, 8, 2000714. [Google Scholar] [CrossRef]
- Lado, J.J.; Zornitta, R.L.; Vázquez Rodríguez, I.; Malverdi Barcelos, K.; Ruotolo, L.A.M. Sugarcane Biowaste-Derived Biochars as Capacitive Deionization Electrodes for Brackish Water Desalination and Water-Softening Applications. ACS Sustain. Chem. Eng. 2019, 7, 18992–19004. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2019, 381, 122736. [Google Scholar] [CrossRef]
- Mohamed, E.F.; El-Hashemy, M.A.; Abdel-Latif, N.M.; Shetaya, W.H. Production of sugarcane bagasse-based activated carbon for formaldehyde gas removal from potted plants exposure chamber. J. Air Waste Manag. Assoc. (1995) 2015, 65, 1413–1420. [Google Scholar] [CrossRef]
- Rath, P.C.; Patra, J.; Huang, H.-T.; Bresser, D.; Wu, T.-Y.; Chang, J.-K. Carbonaceous Anodes Derived from Sugarcane Bagasse for Sodium-Ion Batteries. ChemSusChem 2019, 12, 2302–2309. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Lan, W.; Li, D.; Zhang, A.; Liu, C. Construction of sugarcane bagasse-derived porous and flexible carbon nanofibers by electrospinning for supercapacitors. Ind. Crops Prod. 2021, 170, 113700. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Sarkar, S.; Arya, A.; Gaur, U.K.; Gaur, A. Investigations on porous carbon derived from sugarcane bagasse as an electrode material for supercapacitors. Biomass Bioenergy 2020, 142, 105730. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Lewis, R.; Hreid, T.; Zhang, Z.; Wang, H. Biorefining of sugarcane bagasse to fermentable sugars and surface oxygen group-rich hierarchical porous carbon for supercapacitors. Renew. Energy 2020, 162, 2306–2317. [Google Scholar] [CrossRef]
- Wei, B.; Wei, T.; Xie, C.; Li, K.; Hang, F. Promising activated carbon derived from sugarcane tip as electrode material for high-performance supercapacitors. RSC Adv. 2021, 11, 28138–28147. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, L.; Guo, S.; Jiang, B.; Zhao, D.; Yan, H.; Tian, C.; Fu, H. A hierarchical porous carbon material from a loofah sponge network for high performance supercapacitors. RSC Adv. 2015, 5, 42430–42437. [Google Scholar] [CrossRef]
- Eguchi, T.; Kanamoto, Y.; Tomioka, M.; Tashima, D.; Kumagai, S. Effect of Ball Milling on the Electrochemical Performance of Activated Carbon with a Very High Specific Surface Area. Batteries 2020, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Fang, B.; Wei, Y.Z.; Kumagai, M. Modified carbon materials for high-rate EDLCs application. J. Power Sources 2006, 155, 487–491. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Li, C.; Wang, X.; Wang, K.; Wang, Q. Effect of high-energy ball milling on the microstructure and properties of ultrafine gradient cemented carbides. Int. J. Appl. Ceram. Technol. 2020, 17, 2298–2306. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Manikandan, A.; Subramanian, K.; Kannaiyan, P. Effect of high energy ball milling on particle size and surface area of adsorbents for efficient loading of fertilizer. Asian J. Soil Sci. 2013, 8, 249–254. [Google Scholar]
- Hongo, T.; Yoshino, S.; Yamazaki, A.; Yamasaki, A.; Satokawa, S. Mechanochemical treatment of vermiculite in vibration milling and its effect on lead (II) adsorption ability. Appl. Clay Sci. 2012, 70, 74–78. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Park, S.-J. Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J. Colloid Interface Sci. 2010, 352, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; et al. Hydrophilic Hierarchical Nitrogen-Doped Carbon Nanocages for Ultrahigh Supercapacitive Performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef]
- Jain, A.; Tripathi, S.K. Nano-porous activated carbon from sugarcane waste for supercapacitor application. J. Energy Storage 2015, 4, 121–127. [Google Scholar] [CrossRef]
- Chang, B.; Yang, B.; Guo, Y.; Wang, Y.; Dong, X. Preparation and enhanced supercapacitance performance of porous carbon spheres with a high degree of graphitization. RSC Adv. 2015, 5, 2088–2095. [Google Scholar] [CrossRef]
- Li, B.; Nan, Y.; Zhang, P.; Song, X. Structural characterization of individual graphene sheets formed by arc discharge and their growth mechanisms. RSC Adv. 2016, 6, 19797–19806. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, K.-X.; Li, G.-D.; Chen, J.-S. Hierarchical porous carbon derived from rice straw for lithium ion batteries with high-rate performance. Electrochem. Commun. 2009, 11, 130–133. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Han, S.; Wang, X.; Wang, L.; Yu, W.; Wang, Z. Compositional controls on pore-size distribution by nitrogen adsorption technique in the Lower Permian Shanxi Shales, Ordos Basin. J. Nat. Gas Sci. Eng. 2016, 34, 1369–1381. [Google Scholar] [CrossRef]
- Tran, T.; Phạm, V.T.; Quynh, B.; Thanh Cong, H.; Tam, D.; Thuan, V.; Bach, L.G. Production of Activated Carbon from Sugarcane Bagasse by Chemical Activation with ZnCl2: Preparation and Characterization Study. Res. J. Chem. Sci. 2016, 6, 42–47. [Google Scholar]
- Kumar, A.; Pande, U.; Banerjee, M.K. Effect of high energy ball milling on the structure of iron—Multiwall carbon nanotubes (MWCNT) composite. Adv. Mater. Res. 2017, 6, 245–255. [Google Scholar]
- Bleda-Martínez, M.J.; Lozano-Castelló, D.; Morallón, E.; Cazorla-Amorós, D.; Linares-Solano, A. Chemical and electrochemical characterization of porous carbon materials. Carbon 2006, 44, 2642–2651. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Li, M.; Wang, Y.; Sun, X.; Wang, F.; Yang, R. Soybean Root-Derived Hierarchical Porous Carbon as Electrode Material for High-Performance Supercapacitors in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 33626–33634. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- You, X.; Misra, M.; Gregori, S.; Mohanty, A.K. Preparation of an Electric Double Layer Capacitor (EDLC) Using Miscanthus-Derived Biocarbon. ACS Sustain. Chem. Eng. 2018, 6, 318–324. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakao, H.; Mukai, T.; Katayama, Y.; Miyake, Y. An Experimental Investigation of the Ion Storage/Transfer Behavior in an Electrical Double-Layer Capacitor by Using Monodisperse Carbon Spheres with Microporous Structure. J. Phys. Chem. C 2012, 116, 26791–26799. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Zia, A.I.; Abdul Rahman, M.S.; Mukhopadhyay, S.C.; AlBahadly, I.H.; Yu, P.-L.; Gooneratne, C.; Kosel, J.; Taishan, L. MEMS based impedimetric sensing of phthalates. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013; pp. 855–860. [Google Scholar]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Wang, L.; Mu, G.; Tian, C.; Sun, L.; Zhou, W.; Yu, P.; Yin, J.; Fu, H. Porous Graphitic Carbon Nanosheets Derived from Cornstalk Biomass for Advanced Supercapacitors. ChemSusChem 2013, 6, 880–889. [Google Scholar] [CrossRef]
- Kalpana, D.; Cho, S.H.; Lee, S.B.; Lee, Y.S.; Misra, R.; Renganathan, N.G. Recycled waste paper—A new source of raw material for electric double-layer capacitors. J. Power Sources 2009, 190, 587–591. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, A.M.; Nahm, K.S.; Thomas, S.; Wei, B. Supercapacitors from Activated Carbon Derived from Banana Fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Hou, B. High Specific Surface Area Activated Carbon with Well-Balanced Micro/Mesoporosity for Ultrahigh Supercapacitive Performance. Int. J. Electrochem. Sci. 2016, 11, 9007–9018. [Google Scholar] [CrossRef]
- Ganesan, A.; Mukherjee, R.; Raj, J.; Shaijumon, M.M. Nanoporous rice husk derived carbon for gas storage and high performance electrochemical energy storage. J. Porous Mater. 2014, 21, 839–847. [Google Scholar] [CrossRef]
- Mitravinda, T.; Nanaji, K.; Anandan, S.; Jyothirmayi, A.; Chakravadhanula, V.S.K.; Sharma, C.S.; Rao, T.N. Facile Synthesis of Corn Silk Derived Nanoporous Carbon for an Improved Supercapacitor Performance. J. Electrochem. Soc. 2018, 165, A3369–A3379. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Hu, C.-C.; Wang, C.-C. Effects of pore structure and electrolyte on the capacitive characteristics of steam- and KOH-activated carbons for supercapacitors. J. Power Sources 2005, 144, 302–309. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, K.H.; Shim, W.G.; balathanigaimani, M.S.; Moon, H. Performance of electrochemical double layer capacitors using highly porous activated carbons prepared from beer lees. J. Ind. Eng. Chem. 2011, 17, 450–454. [Google Scholar] [CrossRef]
- Chen, L.; Ji, T.; Brisbin, L.; Zhu, J. Hierarchical Porous and High Surface Area Tubular Carbon as Dye Adsorbent and Capacitor Electrode. ACS Appl. Mater. Interfaces 2015, 7, 12230–12237. [Google Scholar] [CrossRef]
- Mehare, M.D.; Deshmukh, A.D.; Dhoble, S.J. Preparation of porous agro-waste-derived carbon from onion peel for supercapacitor application. J. Mater. Sci. 2020, 55, 4213–4224. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Khosla, K.; Zhu, Z.; Lu, G.Q. Microstructure and electrochemical double-layer capacitance of carbon electrodes prepared by zinc chloride activation of sugar cane bagasse. J. Power Sources 2010, 195, 912–918. [Google Scholar] [CrossRef]









| Sample | SBET (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Davg (nm) |
|---|---|---|---|---|---|
| AC | 2631.9 | 1.2644 | 0.6345 | 0.4814 | 0.60 |
| AC/BM6 | 2592.4 | 1.2680 | 0.6389 | 0.3554 | 0.57 |
| AC/BM12 | 1580.9 | 0.9207 | 0.6318 | 0.3817 | 0.48 |
| AC/BM18 | 1549.7 | 1.0238 | 0.5508 | 0.7017 | 0.50 |
| Sample | Atomic Concentration (%) | O/C | |
|---|---|---|---|
| C1s | O1s | ||
| AC | 84.33 | 15.67 | 0.186 |
| AC/BM6 | 86.73 | 13.27 | 0.153 |
| AC/BM12 | 82.22 | 17.78 | 0.216 |
| AC/BM18 | 79.52 | 20.48 | 0.258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wannasen, L.; Chanlek, N.; Siriroj, S.; Maensiri, S.; Swatsitang, E.; Pinitsoontorn, S. Enhanced Electrochemical Performance of Sugarcane Bagasse-Derived Activated Carbon via a High-Energy Ball Milling Treatment. Nanomaterials 2022, 12, 3555. https://doi.org/10.3390/nano12203555
Wannasen L, Chanlek N, Siriroj S, Maensiri S, Swatsitang E, Pinitsoontorn S. Enhanced Electrochemical Performance of Sugarcane Bagasse-Derived Activated Carbon via a High-Energy Ball Milling Treatment. Nanomaterials. 2022; 12(20):3555. https://doi.org/10.3390/nano12203555
Chicago/Turabian StyleWannasen, Likkhasit, Narong Chanlek, Sumeth Siriroj, Santi Maensiri, Ekaphan Swatsitang, and Supree Pinitsoontorn. 2022. "Enhanced Electrochemical Performance of Sugarcane Bagasse-Derived Activated Carbon via a High-Energy Ball Milling Treatment" Nanomaterials 12, no. 20: 3555. https://doi.org/10.3390/nano12203555
APA StyleWannasen, L., Chanlek, N., Siriroj, S., Maensiri, S., Swatsitang, E., & Pinitsoontorn, S. (2022). Enhanced Electrochemical Performance of Sugarcane Bagasse-Derived Activated Carbon via a High-Energy Ball Milling Treatment. Nanomaterials, 12(20), 3555. https://doi.org/10.3390/nano12203555