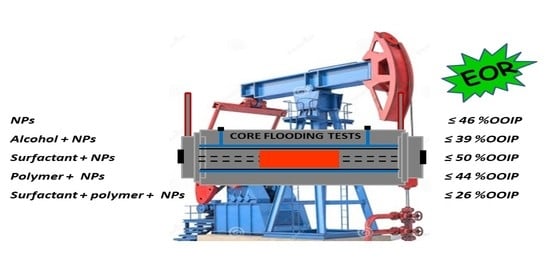
Nanoparticles in Chemical EOR: A Review on Flooding Tests
Abstract
:1. Introduction
2. Review
2.1. Nanoparticles
2.2. Functionalised Nanoparticles
2.3. Alcohol + Nanoparticles
2.4. Surfactant + Nanoparticles
2.5. Polymer + Nanoparticles
2.6. Surfactant + Polymer + Nanoparticles
3. Conclusions
- The literature on the topic is sometimes confusing. Many tests (stability, rheology, IFT, contact-angle, etc.) are carried out, sometimes in the absence of salts and other times in their presence, before the final flooding experiments. As a consequence, the exact composition of the formulation tested in these studies is frequently unclear, especially regarding the presence of salts in the nanofluids. Future works should clearly define the formulation used in oil recovery studies.
- The greatest challenge for EOR in general, and for nano-EOR in particular, is the design of a stable formulation at harsh conditions (temperature and salinity) also considering the presence of divalent ions. This is a difficult task common to all EOR methods, but it is likely more difficult in the presence of nanoparticles. Very few works address the whole problem.
- The simplest and most cost-effective nano-EOR method involves the use of nanofluids consisting only of water or brine and common nanoparticles. Additional oil recoveries achieved with these systems are comparable to those achieved with other EOR methods and justify, in principle, their use to promote oil recovery.
- Even though the number of papers published with simple nanofluids is rather significant, there is not enough information to select the best type and size of nanoparticles according to the application (type of rock, permeability, formation brine, reservoir conditions, etc.). Critically, the number of studies in carbonate rocks is very limited.
- Size and more importantly concentration are key factors that seem to be more critical than the type of nanoparticle for success in practice. The nanofluid concentration must be optimised according to core permeability. Excessive concentration generates aggregation and blocking problems, thus limiting oil extraction and creating pressure problems. Nanoparticle concentration higher than 0.2 wt% is rarely recommended.
- SiO2 nanoparticles are cost-effective and are consequently the most often proposed for the application. Colloidal are preferred to fumed nanoparticles in order to avoid aggregation problems. Al2O3 is sometimes proposed for harsh environments.
- Many experimental studies have confirmed significant adsorption of nanoparticles onto the rock surface during the flooding process. This presents a challenge to nano-EOR due to the associated environmental hazards and operation costs.
- According to several studies, the performance of nanofluids is better when applied as a secondary rather than tertiary EOR method. However, the cost of this injection method is a limiting factor.
- Nanofluid stability is a bottle-neck that sometimes can only be improved using dispersions in alcohols or via the use of different stabilizers.
- Nanoparticles favour disjoining pressure to sweep the oil droplets from rock surfaces. According to the studies presented, the main mechanism is wettability alteration. In certain cases, a reduction in water–oil IFT is also found. However, the addition of a surfactant to the nanofluid drastically enhances this reduction, at the same time as improving or worsening the stability of the nanoparticles.
- To avoid excessive adsorption, cationic surfactants are recommended for carbonate and anionic ones for sandstone rocks. However, a general rule cannot be established regarding the best type of nanoparticles according to surfactant type. More work is required in this line of research.
- Polymers, mixed with nanoparticles or used to functionalise them, are usually employed to improve the stability of nanofluids. Nanoparticles help the polymer to increase aqueous viscosity but also reduce apparent oil viscosity and improve its rheological behaviour.
- The synergy of combining nanoparticles, polymers and surfactants leads to promising formulations for EOR. However, designed formulations are complicated and involve high costs. Moreover, the protocol used to prepare the mixtures, especially in the presence of salts, has to be clearly defined.
- The combination of SAILs with nanoparticles is a niche that must be further explored.
- The flooding equipment used in the tests, as well as the type of core and initial conditions, drastically affect oil recoveries with nanofluids, sometimes leading to very optimistic numbers that should be verified.
- A striking absence of information regarding the costs of nano-EOR methods per incremental bbl, a decisive factor for industrial applications, was noted and needs to be addressed.
- Despite the significant number of laboratory studies carried out, the Technology Readiness Level of nano-EOR is still very low; thus, a lot of effort needs to be made to prove the current system in operational environments.
- In summary, there still exists a need for systematic and rigorous works on EOR with nanoparticles, in order to establish general rules for the best design of nanofluids for practical applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AOR | Additional oil recovery |
| AOS | Alpha olefin sulfonate |
| API | American Petroleum Institute |
| CAPD | Cocamido propyl betaine |
| CMC | Carboxy methyl cellulose |
| CNT | Carbon nanotubes |
| CTAB | Hexadecyltrimethylammonium bromide |
| DTAB | Dodecyltrimethylammonium bromide |
| EOR | Enhanced oil recovery |
| GA | Gum Arabic |
| HAHPAM | Hydrophobic association of partially hydrolysed polyacryamide |
| HPAM | Hydrolysed polyacrylamide |
| IFT | Interfacial tension |
| JGO | Janus graphene oxide |
| MWCNTs | Multi-wall carbon nanotubes |
| OOIP | Original oil in place |
| PAM | Polyacrylamide |
| PSP | Potato peel starch |
| PVP | Polyvinylpyrrolidone |
| r.t. | Room temperature |
| SAILs | Surface-active ionic liquids |
| SEM | Scanning electron microscopy |
| SDBS | Sodium dodecylbenzene sulfonate |
| SDS | Sodium dodecyl sulphate |
| SSW | Synthetic sea water |
| SW | Sea water |
References
- Ahmad, T.; Zhang, D. A critical review of comparative global historical energy consumption and future demand: The story told so far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Sheng, J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Burlington, VT, USA, 2011; pp. 79–567. [Google Scholar] [CrossRef]
- Cheraghian, G. Effects of nanoparticles on wettability: A review on applications of nanotechnology in the enhanced Oil recovery. Int. J. Nanodimension 2015, 6, 443–452. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wang, D.; Jiang, S. Review on enhanced oil recovery by nanofluids. Oil Gas Sci. Technol. 2018, 73, 37. [Google Scholar] [CrossRef] [Green Version]
- Almahfooda, M.; Baia, B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR Applications. J. Petrol. Sci. Eng. 2018, 171, 196–210. [Google Scholar] [CrossRef]
- Afolabi, R.O. Enhanced oil recovery for emergent energy demand: Challenges and prospects for a nanotechnology paradigm shift. Int. Nano Lett. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Allam, N.K. Impact of Nanotechnology on Enhanced Oil Recovery: A Mini-Review. Ind. Eng. Chem. Res. 2019, 58, 16287–16295. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, X.; Tian, F.; Liu, X.; Yuan, J.; Huang, B. Review on nanoparticle-surfactant nanofluids: Formula fabrication and applications in enhanced oil recovery. J. Disper. Sci. Technol. 2020, 43, 745–759. [Google Scholar] [CrossRef]
- Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in Enhanced Oil Recovery. Processes 2020, 8, 1073. [Google Scholar] [CrossRef]
- Nasr, M.S.; Esmaeilnezhad, E.; Choi, H.J. Effect of carbon-based and metal-based nanoparticles on enhanced oil recovery: A review. J. Mol. Liq. 2021, 338, 116903. [Google Scholar] [CrossRef]
- Jafarbeigi, E.; Mohammadidoust, A.; Ranjbar, B. A review on applications of nanoparticles in the enhanced oil recovery in carbonate reservoirs. Petrol. Sci. Technol. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, N.; Zhang, Y.; Wei, M.; Bai, B. Experimental Data Analysis of Nanoparticles for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2019, 58, 12438–12450. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Hendraningrat, L.; Shidong, L.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Petrol. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Effects of the initial rock wettability on silica-based nanofluid-enhanced oil recovery processes at reservoir temperatures. Energy Fuels 2014, 28, 6228–6241. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. A study of water chemistry extends the benefits of using silica-based nanoparticles on enhanced oil recovery. Appl. Nanosci. 2016, 6, 83–95. [Google Scholar] [CrossRef]
- Youssif, M.; El-Maghraby, R.; Saleh, S.M.; Elgibaly, A. Silica nanofluid flooding for enhanced oil recovery in sandstone rocks. Egypt. J. Pet. 2018, 27, 105–110. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Zhou, Y.; Zhang, C. Enhanced oil recovery of low-permeability cores by SiO2 nanofluid. Energy Fuels 2017, 31, 5612–5621. [Google Scholar] [CrossRef]
- Nwufoh, P.; Hu, Z.; Wen, D.; Wang, M. Nanoparticle Assisted EOR during Sand-Pack Flooding: Electrical Tomography to Assess Flow Dynamics and Oil Recovery. Sensors 2019, 19, 3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandio, T.A.; Manan, M.A.; Memon, K.R.; Abbas, G.; Abbasi, G.R. Enhanced Oil Recovery by Hydrophilic Silica Nanofluid: Experimental Evaluation of the Impact of Parameters and Mechanisms on Recovery Potential. Energies 2021, 14, 5767. [Google Scholar] [CrossRef]
- Abhishek, R.; Hamouda, A.A.; Murzin, I. Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Colloids. Surf. A 2018, 555, 397–406. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Nanofluid in hydrophilic state for EOR implication through carbonate reservoir. J. Disper. Sci. Techol. 2014, 35, 1537–1542. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ahmad, Z.; Phung, L.; Kashiwao, T.; Bahadori, A. Evaluation of the ability of the hydrophobic nanoparticles of SiO2 in the EOR process through carbonate rock samples. Petrol. Sci. Techol. 2016, 34, 1048–1054. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Ahmadi, P.; Sharifi, M.; Riazi, M. Wettability modification of oil-wet carbonate reservoirs using silica-based nanofluid: An experimental approach. J. Petrol. Sci. Eng. 2019, 178, 700–710. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Vanani, M.B.; Daryasafar, A.; Aghayari, C. Comparative study of different enhanced oil recovery scenarios by silica nanoparticles: An approach to time-dependent wettability alteration in carbonates. J. Mol. Liq. 2021, 324, 115093. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced heavy oil recovery in sandstone cores using TiO2 nanofluids. Energy Fuels 2014, 28, 423–430. [Google Scholar] [CrossRef]
- Hu, Z.; Azmi, S.M.; Raza, G.; Glover, P.W.J.; Wen, D. Nanoparticle-assisted water-flooding in Berea sandstones. Energy Fuels 2016, 30, 2791–2804. [Google Scholar] [CrossRef]
- Wijayanto, T.; Kurihara, M.; Kurniawan, T.; Muraza, O. Experimental investigation of aluminosilicate nanoparticles for enhanced recovery of waxy crude oil. Energy Fuels 2019, 33, 6076–6082. [Google Scholar] [CrossRef]
- Mohammadi, M.; Moghadasi, J.; Naseri, S. An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. Iran. J. Oil Gas Sci. Technol. 2014, 3, 18–26. [Google Scholar] [CrossRef]
- Jafarnezhad, M.; Giri, M.S.; Alizadeh, M. Impact of SnO2 nanoparticles on enhanced oil recovery from carbonate media. Energy Sources Part A 2017, 39, 121–128. [Google Scholar] [CrossRef]
- Bayat, A.E.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of metal oxide nanoparticles on enhanced oil recovery from limestone media at several temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Moghaddam, R.N.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Le Van, S.; Chon, B.H.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field. J. Ind. Eng. Chem. 2018, 58, 319–327. [Google Scholar] [CrossRef]
- Wahaab, F.A.; Adebayo, L.L.; Adekoya, A.A.; Yusuf, J.Y.; Obalalu, A.M.; Yusuff, A.O.; Alqasem., B. Electromagnetic wave-induced nanofluid-oil interfacial tension reduction for enhanced oil recovery. J. Mol. Liq. 2020, 318, 14378. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Pourafshary, P. Investigation of the effect of engineered water/nanofluid hybrid injection on enhanced oil recovery mechanisms in carbonate reservoirs. J. Petrol. Sci. Eng. 2021, 196, 107662. [Google Scholar] [CrossRef]
- Rayeni, N.S.; Imanivarnosfaderani, M.; Rezaei, A.; Gomari, S.R. An experimental study of the combination of smart water and silica nanoparticles to improve the recovery of asphaltenic oil from carbonate reservoirs. J. Petrol. Sci. Eng. 2022, 208, 109445. [Google Scholar] [CrossRef]
- Li, S.; Hendraningrat, L.; Torsæter, O. Improved Oil Recovery by Hydrophilic Silica Nanoparticles Suspension: 2-Phase Flow Experimental Studies. In Proceedings of the International Petroleum Technology Conference, IPTC-16707, Beijing, China, 26–28 March 2013. [Google Scholar] [CrossRef]
- Aurand, K.R.; Dahle, G.S.; Torsæter, O. Comparison of oil recovery for six nanofluids in Berea sandstone cores. In Proceedings of the International Symposium of the Society of Core Analysts, SCA-A064, Avignon, France, 7–12 September 2014. [Google Scholar]
- El-Diasty, A.I. The potential of nanoparticles to improve oil recovery in bahariya formation, Egypt: An experimental study. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, SPE-174599-MS, Kuala Lumpur, Malaysia, 11–13 August 2015. [Google Scholar] [CrossRef]
- Ragab, A.M.; Hannora, A.E. An experimental investigation of silica nano particles for enhanced oil recovery applications. In Proceedings of the SPE North Africa Technical Conference and Exhibition, SPE-175829-MS, Cairo, Egypt, 14–16 September 2015. [Google Scholar] [CrossRef]
- Alomair, O.A.; Matar, K.M.; Alsaeed, Y.H. Experimental study of enhanced-heavy-oil recovery in Berea sandstone cores by use of nanofluids applications. SPE Reserv. Eval. Eng. 2015, 18, 387–399. [Google Scholar] [CrossRef]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolyzed polyacrylamide used for EOR in high-temperature and high-salinity reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef] [Green Version]
- Sagala, F.; Montoya, T.; Hethnawi, A.; Vitale, G.; Nassar, N.N. Nanopyroxene-based nanofluids for enhanced oil recovery in sandstone cores at reservoir temperature. Energy Fuels 2019, 33, 877–890. [Google Scholar] [CrossRef]
- Cao, J.; Wang, J.; Wang, X.; Zhang, J.; Liu, K.; Wang, Y.; Zhen, W.; Chen, Y. Preparation and characterization of modified amphiphilic nano-silica for enhanced oil recovery. Colloids Surfaces A 2022, 633, 127864. [Google Scholar] [CrossRef]
- Nazarahari, M.J.; Manshad, A.K.; Ali, M.; Ali, J.A.; Shafiei, A.; Sajadi, S.M.; Moradi, S.; Iglauer, S.; Keshavarz, A. Impact of a novel biosynthesized nanocomposite (SiO2@Montmorilant@Xanthan) on wettability shift and interfacial tension: Applications for enhanced oil recovery. Fuel 2021, 298, 120773. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Low-salinity polymeric nanofluid-enhanced oil recovery using green polymer-coated ZnO/SiO2 nanocomposites in the Upper Qamchuqa Formation in Kurdistan Region, Iraq. Energy Fuels 2019, 33, 927–937. [Google Scholar] [CrossRef]
- Rezvani, H.; Riazi, M.; Tabaei, M.; Kazemzadeh, Y.; Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites. Colloids Surfaces A 2018, 544, 15–27. [Google Scholar] [CrossRef]
- Izadi, N.; Koochi, M.M.; Amrollahi, A.; Pourkhalil, M. Investigation of functionalized polyelectrolyte polymer-coated Fe3O4 nanoparticles stabilized in high salinity brine at high temperatures as an EOR agent. J. Petrol. Sci. Eng. 2019, 178, 1079–1091. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.; Xi, J. Mechanisms of nanofluid based modification MoS2 nanosheet for enhanced oil recovery in terms of interfacial tension, wettability alteration and emulsion stability. J. Dispers. Sci. Technol. 2021, 31, 5612–5621. [Google Scholar] [CrossRef]
- Luo, D.; Wang, F.; Zhu, J.; Cao, F.; Liu, Y.; Li, X.; Willson, R.C.; Yang, Z.; Chu, C.-W.; Ren, Z. Nanofluid of graphene-based amphiphilic Janus nanosheets for tertiary or enhanced oil recovery: High performance at low concentration. Proc. Natl. Acad. Sci. USA 2016, 113, 7711–7716. [Google Scholar] [CrossRef] [Green Version]
- Habibi, S.; Jafari, A.; Fakhroueian, Z. Wettability alteration analysis of smart water/novel functionalized nanocomposites for enhanced oil recovery. Pet. Sci. 2020, 17, 1318–1328. [Google Scholar] [CrossRef] [Green Version]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar] [CrossRef] [Green Version]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ efficiency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Onyekonwu, M.E.; Ogolo, N.A. Investigating the Use of Nanoparticles in Enhancing Oil Recovery. In Proceedings of the Nigeria Annual International Conference and Exhibition, SPE-140744, Tinapa-Calabar, Nigeria, 31 July–7 August 2010. [Google Scholar] [CrossRef]
- Roustaei, A.; Moghadasi, J.; Iran, A.; Bagherzadeh, H.; Shahrabadi, A. An experimental investigation of polysilicon nanoparticles’ recovery efficiencies through changes in interfacial tension and wettability alteration. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, SPE-156976, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar] [CrossRef]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Ehsan Eshraghi, S.; Kazemzadeh, Y.; Qahramanpour, M.; Kazemi, A. Investigating effect of SiO2 nanoparticle and sodium-dodecyl-sulfate surfactant on surface properties: Wettability alteration and IFT reduction. J. Pet. Environ. Biotechnol. 2017, 8, 349–353. [Google Scholar] [CrossRef]
- Bagrezaie, M.A.; Pourafsharey, P. Improvement of surfactant flooding performance by application of nanoparticles in sandstone reservoirs. J. Jpn. Petrol. Inst. 2015, 58, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Li, H.; Zhao, M.; Jiao, B. Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. J. Petrol. Sci. Eng. 2017, 153, 283–287. [Google Scholar] [CrossRef]
- Zallaghi, M.; Kharrat, R.; Hashemi, A. Improving the microscopic sweep efficiency of water flooding using silica nanoparticles. J. Pet. Explor. Prod. Technol. 2018, 8, 259–269. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K. Improvement of heavy oil recovery and role of nanoparticles of clay in the surfactant flooding process. Pet. Sci. Technol. 2016, 34, 1397–1405. [Google Scholar] [CrossRef]
- Rezk, M.Y.; Allam, N.K. Unveiling the Synergistic Effect of ZnO Nanoparticles and Surfactant Colloids for Enhanced Oil Recovery. Colloids Interface Sci. Commun. 2019, 29, 33–39. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Han, Y.; Wang, Q.; Wei, X.; Huang, W.; Dai, J.; Song, J.; Yan, H.; Liu, D. Synergistic effects of Janus graphene oxide and surfactants on the heavy oil/water interfacial tension and their application to enhance heavy oil recovery. J. Mol. Liq. 2020, 314, 113791. [Google Scholar] [CrossRef]
- Emadi, S.; Shadizadeh, S.R.; Manshad, A.K.; Rahimi, A.M.; Mohammadi, A.H. Effect of nano silica particles on Interfacial Tension (IFT) and mobility control of natural surfactant (Cedr Extraction) solution in enhanced oil recovery process by nano-surfactant flooding. J. Mol. Liq. 2017, 248, 163–167. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Manteghian, M. Investigation of synergistic effects between silica nanoparticles, biosurfactant and salinity in simultaneous flooding for enhanced oil recovery. RSC Adv. 2019, 9, 20281–20294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Luo, Y.; Lai, R.; Cui, K.; Li, H.; Zhang, Z.; Zhang, Y.; Shi, R. New Technique for Enhancing Oil Recovery from Low-Permeability Reservoirs: The Synergy of Silica Nanoparticles and Biosurfactant. Energy Fuels 2021, 35, 318–328. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Zhong, X.; Sun, W.; Pu, H.; Zhao, J.X. Surfactant-Augmented functional silica nanoparticle based nanofluid for enhanced oil recovery at high temperature and salinity. ACS Appl. Mater. Interfaces 2019, 11, 45763–45775. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lu, J.; Pu, W.; Xie, Q.; Lu, Y.; Du, D.; Yang, X. Synergetic effect between in-situ mobility control and micro-displacement for chemical enhanced oil recovery (CEOR) of a surface-active nanofluid. J. Petrol. Sci. Eng. 2021, 205, 108983. [Google Scholar] [CrossRef]
- Franco, C.A.; Giraldo, L.J.; Candela, C.H.; Bernal, K.M.; Villamil, F.; Montes, D.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Design and Tuning of Nanofluids Applied to Chemical Enhanced Oil Recovery Based on the Surfactant–Nanoparticle–Brine Interaction: From Laboratory Experiments to Oil Field Application. Nanomaterials 2020, 10, 1579. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdollahi, H.; Derikvand, Z.; Hemmati-Sarapardeh, A.; Mosavi, A.; Nabipour, N. Insights into the effects of pore size distribution on the flowing behavior of carbonate rocks: Linking a nano-based enhanced oil recovery method to rock typing. Nanomaterials 2020, 10, 972. [Google Scholar] [CrossRef]
- Kuang, W.; Saraji, S.; Piri, M. A systematic experimental investigation on the synergistic effects of aqueous nanofluids on interfacial properties and their implications for enhanced oil recovery. Fuel 2018, 220, 849–870. [Google Scholar] [CrossRef]
- Asl, H.F.; Zargar, G.; Manshad, A.K.; Takassi, M.A.; Ali, J.A.; Keshavarz, A. Effect of SiO2 nanoparticles on the performance of L-Arg and L-Cys surfactants for enhanced oil recovery in carbonate porous media. J. Mol. Liq. 2020, 300, 112290. [Google Scholar] [CrossRef]
- Rezaei, A.; Riazi, M.; Escrochi, M.; Elhaei, R. Integrating surfactant, alkali and nano-fluid flooding for enhanced oil recovery: A mechanistic experimental study of novel chemical combinations. J. Mol. Liq. 2020, 308, 113106. [Google Scholar] [CrossRef]
- Rezaei, A.; Khodabakhshi, A.; Esmaeili, A.; Razavifar, M. Effects of initial wettability and different surfactant-silica nanoparticles flooding scenarios on oil-recovery from carbonate rocks. Petroleum 2021, in press. [CrossRef]
- Pillai, P.; Saw, R.K.; Singh, R.; Padmanabhan, E.; Mandal, A. Effect of synthesized lysine-grafted silica nanoparticle on surfactant stabilized O/W emulsion stability: Application in enhanced oil recovery. J. Petrol. Sci. Eng. 2019, 177, 861–871. [Google Scholar] [CrossRef]
- Jia, H.; Wu, H.; Wei, X.; Han, Y.; Wang, Q.; Song, J.; Liu, D. Investigation on the effects of AlOOH nanoparticles on sodium dodecylbenzenesulfonate stabilized o/w emulsion stability for EOR. Colloids Surfaces A 2020, 603, 125278. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, G.; Ge, J.; Zhang, J.; Zhang, Q. Investigation of synergy between nanoparticle and surfactant in stabilizing oil-in-water emulsions for improved heavy oil recovery. Colloids Surfaces A 2015, 484, 478–484. [Google Scholar] [CrossRef]
- Pei, H.; Shu, Z.; Zhang, G.; Ge, J.; Jiang, P.; Qin, Y.; Cao, X. Experimental study of nanoparticle and surfactant stabilized emulsion flooding to enhance heavy oil recovery. J. Petrol. Sci. Eng. 2018, 163, 476–483. [Google Scholar] [CrossRef]
- Qin, T.; Goual, L.; Piri, M.; Hu, Z.; Wen, D. Nanoparticle-stabilized microemulsions for enhanced oil recovery from heterogeneous rocks. Fuel 2020, 74, 117830. [Google Scholar] [CrossRef]
- Ali, H.; Soleimani, H.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.R.; Hussain, T.; Adebayo, L.L. Enhanced oil recovery by using electromagnetic-assisted nanofluids: A review. J. Mol. Liq. 2020, 309, 113095. [Google Scholar] [CrossRef]
- Adil, M.; Lee, K.; Mohd Zaid, H.; Ahmad Latiff, N.R.; Alnarabiji, M.S. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). PLoS ONE 2018, 13, e0193518. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Synergistic application of aluminium oxide nanoparticles and oilfield polyacrylamide for enhanced oil recovery. J. Petrol. Sci. Eng. 2019, 182, 106345. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Effect of aluminium oxide nanoparticles on oilfield polyacrylamide: Rheology, interfacial tension, wettability and oil displacement studies. J. Mol. Liq. 2019, 296, 111863. [Google Scholar] [CrossRef]
- Elhaei, R.; Kharrat, R.; Madani, M. Stability, flocculation, and rheological behavior of silica suspension-augmented polyacrylamide and the possibility to improve polymer flooding functionality. J. Mol. Liq. 2021, 322, 114572. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Petrol. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalilinezhad, S.S. Effect of nanoclay on heavy oil recovery during polymer flooding. Petrol. Sci. Technol. 2015, 33, 999–1007. [Google Scholar] [CrossRef]
- Haruna, M.A.; Nourafkan, E.; Hu, Z.; Wen, D. Improved polymer flooding in harsh environments by free-radical polymerization and the use of nanomaterials. Energy Fuels 2019, 33, 1637–1648. [Google Scholar] [CrossRef]
- Haruna, M.A.; Gardy, J.; Yao, G.; Hu, Z.; Hondow, N.; Wen, D. Nanoparticle modified polyacrylamide for enhanced oil recovery at harsh conditions. Fuel 2020, 268, 117186. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsaeter, O. Unlocking the Potential of Metal Oxides Nanoparticles to Enhance the Oil Recovery. In Proceedings of the Offshore Technology Conference-Asia, OTC-24696-MS, Kuala Lumpur, Malaysia, 22–25 March 2014. [Google Scholar] [CrossRef]
- Orodu, K.B.; Afolabi, R.O.; Oluwasijuwomi, T.D.; Orodu, O.D. Effect of aluminum oxide nanoparticles on the rheology and stability of a biopolymer for enhanced oil recovery. J. Mol. Liq. 2019, 288, 110864. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.; Tiwari, P. Silica nanoparticle assisted polymer flooding of heavy crude oil: Emulsification, rheology, and wettability alteration characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Bera, A.; Shah, S.; Shah, M.; Agarwal, J.; Vij, R.K. Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surfaces A 2020, 598, 124833. [Google Scholar] [CrossRef]
- Zhang, H.; Ramakrishnan, T.S.; Nikolov, A.; Wasan, D. Enhanced oil displacement by nanofluid’s structural disjoining pressure in model fractured porous media. J. Colloid Interface Sci. 2018, 511, 48–56. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdi-Khangah, M.; Mohebbi, A.; Tatar, A.; Mohammadi, A.H. Using surface modified clay nanoparticles to improve rheological behavior of Hydrolized Polyacrylamid (HPAM) solution for enhanced oil recovery with polymer flooding. J. Mol. Liq. 2016, 222, 1148–1156. [Google Scholar] [CrossRef]
- Ali, J.A.; Koloc, K.; KhaksarManshad, K.; Stephen, K.D. Emerging applications of TiO2/SiO2/poly(acrylamide) nanocomposites within the engineered water EOR in carbonate reservoirs. J. Mol. Liq. 2021, 322, 114943. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica nanofluids in an oilfield polymer polyacrylamide: Interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Impact of PAM-Grafted nanoparticles on the performance of hydrolyzed polyacrylamide solutions for heavy oil recovery at different salinities. Ind. Eng. Chem. Res. 2019, 58, 9888–9899. [Google Scholar] [CrossRef]
- Son, H.A.; Lee, T. Enhanced Oil Recovery with Size-Dependent Interactions of Nanoparticles Surface-Modified by Zwitterionic Surfactants. Appl. Sci. 2021, 11, 7184. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Zhong, X.; Reagen, S.; Zhang, S.; Sun, W.; Zhao, J.X. Polymer nanoparticles based nano-fluid for enhanced oil recovery at harsh formation conditions. Fuel 2020, 267, 117251. [Google Scholar] [CrossRef]
- Sharma, T.; Kumar, G.S.; Sangwai, J.S. Comparative effectiveness of production performance of Pickering emulsion stabilized by nanoparticle–surfactant–polymerover surfactant–polymer (SP) flooding for enhanced oil recoveryfor Brownfield reservoir. J. Petrol. Sci. Eng. 2015, 129, 221–232. [Google Scholar] [CrossRef]
- Kumar, N.; Gaur, T.; Mandal, A. Characterization of SPN Pickering emulsions for application in enhanced oil recovery. J. Ind. Eng. Chem. 2017, 54, 304–315. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, N.; Saw, R.K.; Mandal, A. Gemini surfactant/polymer/silica stabilized oil-in-water nanoemulsions: Design and physicochemical characterization for enhanced oil recovery. J. Petrol. Sci. Eng. 2019, 183, 106464. [Google Scholar] [CrossRef]
- Pal, N.; Mandal, A. Enhanced oil recovery performance of gemini surfactant-stabilized nanoemulsions functionalized with partially hydrolyzed polymer/silica nanoparticles. Chem. Eng. Sci. 2020, 226, 115887. [Google Scholar] [CrossRef]
- Somoza, A.; Arce, A.; Soto, A. Oil recovery tests with ionic liquids: A review and evaluation of 1-decyl-3-methylimidazolium triflate. Petrol. Sci. 2022, 19, 1877–1887. [Google Scholar] [CrossRef]
- Al-Asadi, A.; Somoza, A.; Arce, A.; Rodil, E.; Soto, A. Nanofluid based on 1-dodecylpyridinium chloride for enhanced oil recovery. Petrol. Sci. 2022, in press. [CrossRef]
- Al-Asadi, A.; Arce, A.; Rodil, E.; Soto, A. Enhanced oil recovery with nanofluids based on aluminum oxide and 1-dodecyl-3-methylimidazolium chloride ionic liquid. J. Mol. Liq. 2022, 363, 119798. [Google Scholar] [CrossRef]
| Nanoparticles | Concentration (wt%) | T (°C) | Dispersion Media | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 0.01–0.1 | 20 | 3.0 wt% NaCl | North Sea | Berea sandstone (low-permeability) | Ø: 13.93–15.02% µ: 9–35 mD | 0–6.14 | [18] |
| SiO2 | 0.01–0.1 | 20 | 3.0 wt% NaCl | North Sea | Berea sandstone (high-permeability) | Ø: 20.01–23.20% µ: 156–392 mD | 4.26–5.32 | [18] |
| SiO2 | 0.05 | 25 50 80 | 3.0 wt% NaCl | Light | Berea sandstone | Ø: 15–19% µ: 100–600 mD | 0.81–5.86 1 3.91–10.87 1 6.02–10.11 1 | [19] |
| SiO2 | 0.05 | r.t. | SSW | North Sea | Berea sandstone | Ø: 15–17 % µ: 100–600 mD | 1.98–18.52 1 | [20] |
| SiO2 | 0.01–0.5 | r.t. | 3.0 wt% NaCl | North Sea | Sandstone | Ø: 19.4–21.7 % µ: 285–587 mD | ≤13.3 | [21] |
| SiO2 | 5 × 10−4–0.003 | 50 | 7500 mg/L NaCl | Shengli oilfield | Feldspar (53%) | Ø: 9.35−11.95% µ: 0.68−0.95 mD | 4.48–10.33 1 | [22] |
| SiO2 | 0.02–0.1 | r.t. | 2–4 wt% NaCl | Paraffin | Sandstone | Ø: 20.72–27.65% µ: 465–603 mD | 6.58–11.2 | [24] |
| SiO2 | 0.01–0.1 | r.t. | 3 wt% NaCl | North Sea | Sandstone | Ø: 20.01–23.20% µ: 156–392 mD | 4.26–5.32 | [41] |
| SiO2 Fumed/colloidal | 0.01–0.1 | r.t. | Synthetic North Sea brine 3 | North Sea | Sandstone | Ø: 17.8–20.8% µ: 285–438 mD | 2.36–11.76 | [42] |
| SiO2 | 0.01–3 | r.t. | Water 4 | Mineral oil | Bahariya sandstone | Ø: 26% µ: 378.73 mD | 40–79 2 | [43] |
| SiO2 | 0.1 | r.t. | Water 4 | Mineral oil | Sandstone | Ø: 29.87–30.75% µ: 575.82–642.65 mD | 65.36–77.94 2 | [44] |
| SiO2 | 0.01–0.1 | r.t. | 3 wt% NaCl | Heavy | Berea sandstone | Ø: 17.4–20.3% µ: 60 mD | 28.56–38.57 2 | [45] |
| SiO2 Aerosil 200 | 0.05–1 | 100 | Water 4 | Iranian | Carbonate | Ø: 14.379–15.823% µ: 1.0119–1.9552 mD | 57.23–65.23 2 | [26] |
| SiO2 Aerosil R 816 | 0–1 | 100 | Water 4 | Iranian | Carbonate | Ø: 13.268–14.949% µ: 1.0347–1.2446 mD | 55.45–80.2 2 | [27] |
| SiO2 | 0.005 | 26 40 50 60 | Water 4 | Malaysian | Limestone grains | Ø: 41.1–43.2%. µ: 220–240 mD | 2.0 2.5 2.8 2.9 | [35] |
| TiO2 Anatase | 0.01 | 75 | 5000 ppm NaCl | Heavy | Sandstone | Ø: 23.7 % µ: 84 mD | 80 2 | [30] |
| TiO2 Rutile | 0–0.05 | r.t. | 0.1 mol/dm 3 NaCl | Mineral oil | Berea sandstone | Ø: 20.82–21.20% µ: 98.73–195.46 mD | 30.3–41.8 2 | [31] |
| TiO2 | 0.01–0.1 | r.t. | 3 wt% NaCl | Heavy | Berea sandstone | Ø: 20.0–24.0% µ: 60 mD | 15.69–34.42 2 | [45] |
| TiO2 | 0.005 | 26 40 50 60 | Water4 | Malaysian | Limestone grains | Ø: 42.3–43.3%. µ: 203–236 mD | 3.0 4.1 5.2 6.6 | [35] |
| Aluminosilicate | 10−4–0.002 | 60 | Formation brine Langgak field | Indonesia | Bentheimer sandstone | Ø: 19.33–20.37% µ: 316.93–633.76mD | 4.44–15.59 | [32] |
| Al2O3 | 0.01–0.1 | r.t. | 3 wt% NaCl | Heavy | Berea sandstone | Ø: 19.7–20.4% µ: 60 mD | 32.32–38.70 2 | [45] |
| γ-Al2O3 | 0.5 | r.t. | Water 4 | Ahwaz oilfield | Carbonate | Ø: 17.3% µ: 0.807 mD | 11.25 | [33] |
| Al2O3 | 0.005 | 26 40 50 60 | Water 4 | Malaysian | Limestone grains | Ø: 41.8–43.1%. µ: 205–230 mD | 4.5 5.4 7.0 9.9 | [35] |
| SnO2 | 0–0.5 | r.t. | SSW | Ahwaz oilfield | Carbonate | Ø: 18% µ: 0.22 mD | 39–61 1,2 | [34] |
| NiO | 0.01– 0.1 | r.t. | 3 wt% NaCl | Heavy | Berea sandstone | Ø: 19.0–21.8% µ: 60 mD | 31.45–36.20 2 | [45] |
| SiO2 + Al2O3 (50 wt%) | 0.01– 0.1 | r.t. | 3 wt% NaCl | Heavy | Berea sandstone | Ø: 20.2–20.6% µ: 60 mD | 24.85–42.29 2 | [45] |
| SiO2 + Al2O3 (50 wt%) | 0.05 | r.t. 80 | 16 wt% NaCl | Heavy | Berea sandstone | Ø: 16.1–20.7% µ: 60 mD | 29.36 2 56.72 2 | [45] |
| Magnetite +Magnetic Field | 0.8 | r.t. | 5000 ppm NaCl3 | Van Gogh/Kuwait | Berea sandstone | Ø: 18.94–21.51%. µ: 60–82 mD | 6.32–15.38 1 8.57–16.20 1 | [37] |
| Fe3O4 +Magnetic Field | 0.05 | r.t. | 11,000 ppm NaCl | Light | Berea sandstone | 15.8 29.4 | [38] | |
| SiO2 +Smart Water | 0.1 | 85 | Formation brine + SO42− | Iranian | Carbonate (Dolomite) | Ø: 19.69% µ: 1.0 mD | ~11.3 | [39] |
| SiO2 +Smart Water | 0.05 | 60 | Water + MgCl2 + CaCl2 + Na2SO4 | Iranian (asphaltenic) | Carbonate | 45.6 | [40] |
| Nanoparticles | Concentration (wt%) | T (°C) | Dispersion Media | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|---|
| Nano-hybrid SiO2-HAHPAM | 0.5 HAHPAM/ 0.5 Silica | 85 | Synthetic brine with divalent ions | Shengli | Silica sand | Ø: 32.6 % µ: 1498 mD | 10.57 | [46] |
| Pyroxenes | 0.005 | 60 | 2 wt% NaCl | Canada | Berea sandstone | Ø: 19.2 % µ: 63 mD | 10.57 | [47] |
| Amphiphilic silica | 0.1 | 90 | Water 1 | Paraffin:Kerosene 10:3 | Sandstone | Ø: 20.7–21.4 % µ: 50–800 mD | 2.6–10.3 | [48] |
| SiO2@Montmorilant @Xanthan | 0.1 | 60 | Water 1 | Gachsaran oilfield | Sandstone | Ø: 15.32 % µ: 30 mD | 15.79 | [49] |
| SiO2@Montmorilant @Xanthan | 0.025 | 60 | Water 1 | Gachsaran oilfield | Carbonate | Ø: 12.82 % µ: 8.23 mD | 11.72 | [49] |
| ZnO/SiO2/ Xanthan | 0.2 | 75 | Dilute SW | Medium | Carbonate | Ø: 16.85 % µ: 13.15 mD | 19.28 | [50] |
| Fe3O4@Chitosan | 0–0.03 | r.t. | SW 2 | Iranian | Carbonate sand | 56.7–67.5 3 | [51] | |
| Polymer-citrate-coated Fe3O4 | 0.02–0.04 | 85 | SSW (mixture of formation water and SW) | Iranian | Silane glass beads | Ø: 23–25% µ: 320–340 mD | 12.2–19.7 | [52] |
| Polymer-citrate-coated Fe3O4 | 0.04 | 85 | SSW (mixture of formation water and SW) | Iranian | Carbonate | Ø: 17.8% µ: 34.12 mD | 28 | [52] |
| KH550-MoS2 | 0.005 | 55 | Synthetic formation brine | Changqing oilfield | Chloritization and illite | Ø: 36.22–38.1% µ: 7.8–8.4 mD | 14 | [53] |
| Graphene-based amphiphilic Janus Nanosheets | 0.005–0.01 | r.t. | 4 wt% NaCl + 1 wt% CaCl2 | China oilfield | Sandstone sand-packs | Ø: 24.8–27.9% µ: 44.5–132 mD | 6.7–15.2 | [54] |
| Amine/organosiloxane @Al2O3/SiO2 + Smart Water | 0.005 | r.t. | SSW +Ca2+/SO42− | Medium | Carbonate | Ø: 8.57−11.50% µ: 0.54−0.59 mD | 3–7.5 | [55] |
| Nanoparticles | Concentration (g/L) | Dispersion Media | T (°C) | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|---|
| SiO2 Aerosil R 816 | 3 | Ethanol | r.t. | Light Intermediate | Sandstone | Ø: 18.5% µ: 102 mD | 25.43 14.55 | [57] |
| Hydrophobic polysilicon | 3 | Ethanol | r.t. | Light Intermediate | Sandstone | Ø: 31.29–31.64% µ: 513.59–1476.18 mD | 36.67 29.01 | [58] |
| Hydrophobic polysilicon | 4 | Ethanol | r.t. | Iranian | Sandstone | Ø: 17% µ: 186 mD | 32.20 | [59] |
| Neutrally wet polysilicon | 3 | Ethanol | r.t. | Light Intermediate | Sandstone | Ø: 30.88–31.78% µ: 791.23–939.24 mD | 38.75 29.23 | [58] |
| Neutrally wet polysilicon | 4 | Ethanol | r.t. | Iranian | Sandstone | Ø: 17% µ: 186 mD | 28.57 | [59] |
| SiO2 -treated by silane | 1.5 | Propanol | r.t. | Iranian | Sandstone | Ø: 17.34% µ: 108.21 mD | 22.5 | [60] |
| Al2O3 | 1.5 | Propanol | r.t. | Iranian | Sandstone | Ø: 17.45% µ: 110.40 mD | 20.2 | [60] |
| Fe2O3 | 1.5 | Propanol | r.t. | Iranian | Sandstone | Ø: 18.12% µ: 109.32 mD | 17.3 | [60] |
| Nanofluid | T (°C) | Dispersion Media | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|
| 0.1 wt% SiO2 2150 ppm SDS | 30 | Diluted reservoir brine 2000 ppm 1 | Iranian | Sandstone | Ø: 20% µ: 200 mD | 80 2 | [61] |
| 0.1 wt% SiO2 (Aerosil 200) 2500 ppm SDS | 38 | Water | Iranian | Sandstone | Ø: 15–16% µ: 40-60 mD | 94.7 2 | [62] |
| 0.2 wt% SiO2 0.2 wt% SDS | 20 | Water | Tahe oilfield | Sandstone | Ø: µ: 0.051 μm 2 | 9.11 3 | [63] |
| 0.2 wt% SiO2 (Aerosil 300) 400 ppm SDS | 26 | Synthetic brine with divalent ions | Azadeghan | Sandstone | Ø: 18.2 µ: 158.35 mD | 14.5 | [64] |
| 2000–5000 ppm SiO2 (Aerosil 300) 2000 mg/L SDS | r.t. | Water | 26 cp (25 °C) | Sandstone sand-packs | Ø: 21.34 µ: 367.96 mD | 15.86–17.87 3,4 | [65] |
| 2000–10000 ppm SiO2 (Aerosil R 816) 2000 mg/L SDS | r.t. | Water | 26 cp (25 °C) | Sandstone sand-packs | Ø: 21.34 µ: 367.96 mD | 18.26–20.41 3,4 | [65] |
| 0.5 wt% Nano-clay 1800 ppm SDS | r.t. | Water 5 | Iranian | Sandstone | Ø: 18.3–18.5 µ: 281-285 mD | 52.3 2 | [66] |
| 0.05 wt% ZnO 0.2 wt% SDS | r.t. | Water | Dodecane | Sandstone | Ø: 26.65 µ: 15.74 mD | ~15 | [67] |
| 100 mg/L Janus graphene oxide (400) 1000 mg/L DTAB | 60 | Formation brine | Bohai oilfield | Artificial core | Ø: 37.02 µ: 506 mD | 19.5 3 | [68] |
| 1000 ppm SiO2 5 wt% Cedr extract | 25 | 100,000 ppm NaCl | Reservoir | Sandstone | Ø: 15.4 µ: 1.9 mD | 74 2 | [69] |
| 97.5 ppm SiO2 110.8 ppm Rhamnolipid | 80 | 1.17 wt% NaCl 1 | North Azadegan oilfield | Carbonate | Ø: 14.1 µ: 1.45 mD | 5.1 | [70] |
| 10–500 mg/L SiO2 10–40 mg/L Rhamnolipid | 55 | 3 wt% NaCl | Xinjiang oilfield | Berea sandstone | Ø: 16.45–19.87 µ: 0.0053-3.19 mD | 4–25 | [71] |
| 0.05 wt% SiO2-NH2 0.2 wt% Soloterra 964 | 65 | 15 wt% NaCl | Bakken | Berea sandstone | Ø: 18.96 µ: 0.091 μm 2 | 22.37 3 | [72] |
| 500 mg/L Amine-terminated SiO2 4000 mg/L Laurel anolamide | 30 | Formation brine | Xinjiang Oilfield | Three-layer artificial | Ø: 17.6 µ: 100/200/500 mD 50/100/300 mD | 26.4–29.2 3 | [73] |
| 0.01 wt% SiO2 0.01 wt% Alpha olefin sulfonate | r.t. | 18 wt% NaCl | Iranian | Carbonate | Ø: 13.48-15.73 µ: 1.05-1.51 mD | 2.5–8.6 3 | [75] |
| 0.1 wt% SiOx 0.1 wt% Linear alcohol, C9-11, ethoxylate | 60 | 1mM NaCl | Crude oil | Berea Sandstone | Ø: 23.6 µ: 173.2 mD | ~55 2 | [76] |
| 0.1 wt% SiOx 0.1 wt% Linear alcohol, C9-11, ethoxylate | 60 | 1mM NaCl | Crude oil | Edward carbonate | Ø: 24.23 µ: 23.61 mD | ~29 2,6 | [76] |
| 1000 ppm SiO2 2000 ppm Lauroyl-arginine | Water 5 | Kupal oilfield | Carbonate | Ø: 13.16 µ: 10.88 mD | 13.1 | [77] | |
| 1000 ppm SiO2 4500 ppm Lauroyl-cysteine | Water 5 | Kupal oilfield | Carbonate | Ø: 9.38 µ: 8.28 mD | 12.7 | [77] | |
| 1000 ppm SiO2 250 ppm Cocamido propyl betaine | r.t. | 19.8 wt% NaCl | Iranian | Carbonate (Dolomite) | Ø: 19.4 µ: 8.4 mD | 12.2 | [78] |
| 0.1 wt% SiO2 0.03 wt% Linear alkylbenzene sulfonic acid | r.t. | 20 wt% NaCl | Iranian | Carbonate | Ø: 19-23 µ: 13-16 mD | ~ 4.3–5 | [79] |
| 1 wt% Lysine-grafted silica 8000 ppm Jatropha oil derived 7 | 75 | 2 wt% NaCl | Crude oil | Sand-packs | Ø: 28.55–31.21 µ: 1.16-1.56 mD | 33.6 3 | [80] |
| 1 wt% Aluminium oxide hydroxide 3mM Sodium dodecylbenzene sulfonate Water:dodecane ratio 1:1 7 | 60 | Water 5 | Jidong oilfield | Artificial core | Ø: 36–42 µ: ~500 mD | 33 3 | [81] |
| 0.4 wt% SiO2 0.1 wt% Hexadecyltrimethylammonium bromide Water:biodiesel ratio 9:1 7 | 50 | 0.5 wt% NaCl | Shengli oilfield | Man-made | Ø: 18.1-24.6 µ: 100-1100 mD | 17.40-50.01 | [82] |
| 0.01 wt% SiO2 Triton X-100: n-dodecul β-d-maltoside: d-limonene:2-propanol 2:2:2:0.8 7 | 60 | 1M CaCl2 | Gibbs oilfield | Arkose sandstone | Ø: 16.8–17 µ: 26-32 mD | 34.3 | [84] |
| 0.1 wt% ZnO 0.025 wt% Sodium dodecylbenzene sulfonate + Magnetic Field | 95 | 3 wt% NaCl | Tapis oilfield | Sandstone sand-packs | Ø: 35.40–37.37 µ: 267–284 mD | 13.82–15.25 3 13.98–16.05 3 | [86] |
| Nanofluid | T (°C) | Dispersion Media | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|
| 0.1 wt% SiO2 0.2 wt% HPAM | 90 | Water 1 | Sarawak oilfield | Sandstone | Ø: 15.25–15.31% µ: 165.6–169.1 mD | 33 2 | [87] |
| 0.05–0.1 wt% SiO2 0.2 wt% HPAM | r.t. | Water 1 | Iranian | Sandstone | Ø: 18.65–19.30% µ: 10.21–12.90 mD | 6.28–7.97 | [89] |
| 0.1 wt% Al2O3 0.2 wt% HPAM | 90 | 3.41 wt% NaCl | Sarawak oilfield | Sandstone | Ø: 15.25–15.31% µ: 165.6-169.1 mD | 37.6 2 | [87] |
| 1.9–2.5 wt% TiO2 3150 ppm HPAM | r.t. | Water 1 | Iranian | Sandstone | Ø: 18.2–18.4% µ: 281–283 mD | 40.4–43.3 2 | [90] |
| 0.9 wt% Nano-clay 3150 ppm HPAM | r.t. | Water 1 | Iranian | Sandstone | Ø: 18.7% µ: 284 mD | ~42 | [91] |
| 1000 ppm MWCNTs 1000 ppm Acrylamide co/terpolymers | 85 | API Alkaline pH | Mineral oil | Ottawa sand-pack | Ø: 38.28% µ: 95 mD | 14.8 10.8 | [92] |
| 0.1 wt% Amino-modified SiO2/Acrylamide copolymer nanocomposite | 85 | API Formation brine | Mineral oil | Ottawa sand-pack | Ø: 37.9% µ: 95 mD | 17.46 2 16.20 2 | [93] |
| 0.05 wt% Al2O3 1 wt% Polyvinylpyrrolidone | r.t. | 3 wt% NaCl | North Sea | Berea sandstone | Ø: 14.71% µ: 330 mD | 13.34 2 | [94] |
| 0.05 wt% TiO2 1 wt% Polyvinylpyrrolidone | r.t. | 3 wt% NaCl | North Sea | Berea sandstone | Ø: 14.96 % µ: 118 mD | 20.00 2 | [94] |
| 0.5–1.5 wt% Al2O3 5.0 wt% Potato peel starch | r.t. | Water 1 | Niger Delta | Niger Delta Berea sandstone | Ø: 22.58–26.70% µ: 291.3–293.1 mD | 10.22–12.44 3 | [95] |
| 1.33 wt% Al2O3 3.0–5.0 wt% Gum Arabic | r.t. | Water 1 | Niger Delta | Niger Delta Berea sandstone | Ø: 17.89–18.56% µ: 251.7–278.8 mD | 5.61-7.81 | [95] |
| 0.1–0.5 wt% SiO2 1000–5000 ppm Xanthan gum | 30 80 | Synthetic formation brine | Heavy | Berea sandstone | Ø: 25.1–26.5% µ: 746–1002 mD | 16.29-20.82 2 18.44 2 | [96] |
| 0.2 wt% SiO2 4000 ppm Guar gum | 50 | Water 1 | Light | Sandstone | Ø: 16.3% µ: 204 mD | 44.3 2 | [97] |
| 0.1 wt% Surface-modified montmorillonite 2000 ppm HPAM | r.t. | Water 1 | Darquain oilfield | Carbonate sand-pack | Ø: 23.3% µ: 294 mD | 33 4 | [99] |
| 500–1500 ppm of TiO2/SiO2/poly(acrylamide) nanocomposite + Smart Water | 75 | Different smart waters | Iranian | Carbonate | Ø: 7.75–9.65% µ: 3.60–4.20 mD | 7.4–10.5 | [100] |
| Nanofluid | T (°C) | Dispersion Media | Oil | Rock Type | Rock Properties | AOR (%OOIP) | Ref |
|---|---|---|---|---|---|---|---|
| 0.5–2.0 wt% SiO2 0.14 wt% SDS 1000 ppm HPAM | 30 90 | Water 1 | Medium | Silica sand-pack | Ø: 29.77 ± 1.34% µ: 1006±86.50 mD | 19.25–24.68 2 21.82 2 | [101] |
| 0.2–0.4 wt% PAM-grafted TiO2 0.1 wt% SDS 0.4 wt% HPAM | 25 | 0–3 wt% NaCl | Heavy | Sand-pack | Ø: 34–37% µ: 5276.4–6111.1 mD | ~69–86 2,3 | [102] |
| 0.5 wt% Colloidal SiO2 0.1 wt% 3-(N,N-dimethylmyristylammonio)propanesulfonate 0.5 wt% PSS-co-MA | 25 | 3 wt% NaCl | Silicone | Sandstone | Ø: 19–22% µ: 138-154 mD | 74.2–78.2 3 | [103] |
| 0.08 wt% Polymer nanoparticles 0.1 wt%Betaine surfactant | 80 | Synthetic formation brine | Bakken | Berea sandstone | Ø: 19.22 % µ: 0.08859 μm 2 | 19.95 2 | [104] |
| 1.0 wt% SiO2 1.0 wt% PAM 0.22 wt% SDS-Detergent Water:oil ratio 3:1 4 | 40–90 | Water 1 | Tarapur oilfield | Berea sandstone | Ø: 20.04–22.85 % µ: 498–632 mD | 17.49–21 2 | [105] |
| 1.0 wt% Clay 1.0 wt% PAM 0.22 wt% SDS-Detergent Water:oil ratio 3:1 4 | 40–90 | Water 1 | Tarapur oilfield | Berea sandstone | Ø: 20.17–23.05 % µ: 584–703 mD | 17.25–20.12 2 | [105] |
| 0.25 wt% SiO2 1.5 wt% Carboxy methyl cellulose 825 ppm SDBS Water:oil ratio 9:1 4 | r.t. | Water 1 | Light mineral | Sand pack | Ø: 31.48% µ: 1247 mD | 24.81 2 | [106] |
| 0.025 wt% SiO2 0.05 wt% HPAM 0.1 wt% N,N′-bis((dimethyltetradecyl)-1,6-hexanediammonium bromide) Water:heptane ratio 9:1 4 | r.t. | Water 1 | Ahmedabad oilfield | Sandstone | Ø: 17–18% µ: 350-400 mD | 26.25 2 | [107] |
| 0.05 wt% Al2O3 0.5 wt% 1-Dodecylpyridinium chloride 1 wt% Polyvinylpyrrolidone | r.t. | 0.5 wt% NaCl | Light | Carbonate | Ø: 18.08% µ: 22.02 mD | 11.96 | [110] |
| 0.05 wt% Al2O3 0.05 wt% 1-dodecyl-3-methylimidazolium chloride 1 wt% Polyvinylpyrrolidone | r.t. | 5 wt% NaCl | Light | Carbonate | Ø: 14.57% µ: 13.97 mD | 14.8 | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asadi, A.; Rodil, E.; Soto, A. Nanoparticles in Chemical EOR: A Review on Flooding Tests. Nanomaterials 2022, 12, 4142. https://doi.org/10.3390/nano12234142
Al-Asadi A, Rodil E, Soto A. Nanoparticles in Chemical EOR: A Review on Flooding Tests. Nanomaterials. 2022; 12(23):4142. https://doi.org/10.3390/nano12234142
Chicago/Turabian StyleAl-Asadi, Akram, Eva Rodil, and Ana Soto. 2022. "Nanoparticles in Chemical EOR: A Review on Flooding Tests" Nanomaterials 12, no. 23: 4142. https://doi.org/10.3390/nano12234142
APA StyleAl-Asadi, A., Rodil, E., & Soto, A. (2022). Nanoparticles in Chemical EOR: A Review on Flooding Tests. Nanomaterials, 12(23), 4142. https://doi.org/10.3390/nano12234142