High Performance 0D ZnO Quantum Dot/2D (PEA)2PbI4 Nanosheet Hybrid Photodetectors Fabricated via a Facile Antisolvent Method
Abstract
:1. Introduction
2. Materials and Methods
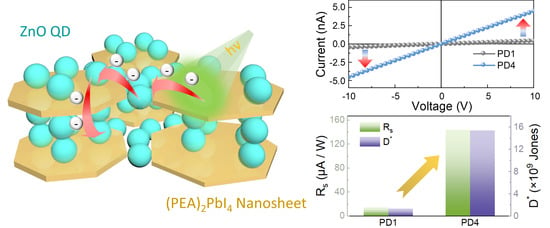
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghosh, S.; Kumar, H.; Mukhopadhyay, B.; Chang, G.-E. Design and Modeling of High-Performance DBR-Based Resonant-Cavity-Enhanced GeSn Photodetector for Fiber-Optic Telecommunication Networks. IEEE Sens. J. 2021, 21, 9900–9908. [Google Scholar] [CrossRef]
- Dong, T.; Simões, J.; Yang, Z. Flexible Photodetector Based on 2D Materials: Processing, Architectures, and Applications. Adv. Mater. Interfaces 2020, 7, 1901657. [Google Scholar] [CrossRef]
- Bartolo-Perez, C.; Chandiparsi, S.; Mayet, A.S.; Cansizoglu, H.; Gao, Y.; Qarony, W.; AhAmed, A.; Wang, S.-Y.; Cherry, S.R.; Saif Islam, M.; et al. Avalanche Photodetectors with Photon Trapping Structures for Biomedical Imaging Applications. Opt. Express 2021, 29, 19024. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Wang, Z. Enhanced Performances of P-Si/N-ZnO Self-Powered Photodetector by Interface State Modification and Pyro-Phototronic Effect. Nano Energy 2020, 71, 104630. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, W.; Yang, W.; He, J.; Fang, X. Recent Progress of Heterojunction Ultraviolet Photodetectors: Materials, Integrations, and Applications. Adv. Funct. Mater. 2020, 30, 1909909. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Zhang, Z.; Li, J.; Uddin, Z.; Zheng, Y.; Shao, Y.; Yuan, Y.; Yang, B. Defect Passivation via Additive Engineering to Improve Photodetection Performance in CsPbI2Br Perovskite Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 56358–56365. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, G.; Chen, C.; Li, Y.; Xu, M.; Wang, T.; Zhao, G.; Zhang, L. Perovskite CsPbBr3 Crystals: Growth and Applications. J. Mater. Chem. C 2020, 8, 6326–6341. [Google Scholar] [CrossRef]
- Loi, H.; Cao, J.; Guo, X.; Liu, C.; Wang, N.; Song, J.; Tang, G.; Zhu, Y.; Yan, F. Gradient 2D/3D Perovskite Films Prepared by Hot-Casting for Sensitive Photodetectors. Adv. Sci. 2020, 7, 2000776. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, Z.; Liu, H.; Liu, X.; Chen, J.; Fang, X. High-Performance Two-Dimensional Perovskite ca2Nb3O10 UV Photodetectors. Nano Lett. 2020, 21, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Z.-Z.; Li, Y.; Zhang, F.; Chen, X.; Shi, Z.-F. Low-Dimensional Phases Engineering for Improving the Emission Efficiency and Stability of Quasi-2D Perovskite Films*. Chin. Phys. B 2021, 30, 067802. [Google Scholar] [CrossRef]
- Yue, Y.; Li, M.; Li, H.; Chai, N.; Dong, Y.; Li, Z.; Chen, X.; Wang, X. One-Step Anti-Solvent Associated Method for High Performance Two-Dimensional Perovskite Photodetectors Fabrication at Low Temperature. Chem. Eng. J. 2022, 441, 135997. [Google Scholar] [CrossRef]
- Cheng, B.; Li, T.-Y.; Wei, P.-C.; Yin, J.; Ho, K.-T.; Retamal, J.R.D.; Mohammed, O.F.; He, J.-H. Layer-Edge Device of Two-Dimensional Hybrid Perovskites. Nat. Commun. 2018, 9, 5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekshahi Byranvand, M.; Behboodi-Sadabad, F.; Alrhman Eliwi, A.; Trouillet, V.; Welle, A.; Ternes, S.; Hossain, I.M.; Khan, M.R.; Schwenzer, J.A.; Farooq, A.; et al. Chemical Vapor Deposited Polymer Layer for Efficient Passivation of Planar Perovskite Solar Cells. J. Mater. Chem. A 2020, 8, 20122–20132. [Google Scholar] [CrossRef]
- Liang, Y.; Shang, Q.; Wei, Q.; Zhao, L.; Liu, Z.; Shi, J.; Zhong, Y.; Chen, J.; Gao, Y.; Li, M.; et al. Lasing from Mechanically Exfoliated 2D Homologous Ruddlesden–Popper Perovskite Engineered by Inorganic Layer Thickness. Adv. Mater. 2019, 31, 1903030. [Google Scholar] [CrossRef] [PubMed]
- Zanca, C.; Piazza, V.; Agnello, S.; Patella, B.; Ganci, F.; Aiello, G.; Piazza, S.; Sunseri, C.; Inguanta, R. Controlled Solution-Based Fabrication of Perovskite Thin Films Directly on Conductive Substrate. Thin Solid Film. 2021, 733, 138806. [Google Scholar] [CrossRef]
- Jha, S.; Hasan, M.; Khakurel, N.; Ryan, C.A.; McMullen, R.; Mishra, A.; Malko, A.V.; Zakhidov, A.A.; Slinker, J.D. Electrochemical Characterization of Halide Perovskites: Stability & Doping. Mater. Today Adv. 2022, 13, 100213. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Dini, D. Electrodeposition as a Versatile Preparative Tool for Perovskite Photovoltaics: Aspects of Metallization and Selective Contacts/Active Layer Formation. Sol. RRL 2022, 6, 2100993. [Google Scholar] [CrossRef]
- Swartwout, R.; Hoerantner, M.T.; Bulović, V. Scalable Deposition Methods for Large-Area Production of Perovskite Thin Films. Energy Environ. Mater. 2019, 2, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Wan, Y.; Li, H.; Fang, S.; Huang, F.; Zhou, B.; Jiang, K.; Tung, V.; Li, L.-J.; Shi, Y. Two-Dimensional Cs2AgBiBr6/WS2 Heterostructure-Based Photodetector with Boosted Detectivity via Interfacial Engineering. ACS Nano 2022, 16, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Akram, J.; Hussain, S.; Chen, J.; Qasim, K.; Zhang, W.; Lei, W. High-Performance Photodetector Based on a Graphene Quantum Dot/CH3NH3PbI3 Perovskite Hybrid. ACS Appl. Electron. Mater. 2019, 2, 230–237. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Liu, S.; Li, M.; Wen, X.; Lee, J.; Lin, S.; Li, M.-Y.; Lu, H. High Performance Hybrid MXene Nanosheet/CsPbBr3 Quantum Dot Photodetectors with an Excellent Stability. J. Alloys Compd. 2022, 895, 162570. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Ma, N.; Cao, K.; Zhang, W.; Chen, J.; Wang, D.; Gao, Z.; Xu, F.; Jiang, K. Single-Crystal-like ZnO Mesoporous Spheres Derived from Metal Organic Framework Delivering High Electron Mobility for Enhanced Energy Conversion and Storage Performances. Electrochim. Acta 2019, 305, 474–483. [Google Scholar] [CrossRef]
- Shen, K.; Li, X.; Xu, H.; Wang, M.; Dai, X.; Guo, J.; Zhang, T.; Li, S.; Zou, G.; Choy, K.-L.; et al. Enhanced Performance of ZnO Nanoparticle Decorated All-Inorganic CsPbBr3 Quantum Dot Photodetectors. J. Mater. Chem. A 2019, 7, 6134–6142. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yu, X.; Zhu, Y.; Liu, S.; Wen, X.; Lu, H.; Wang, C.; Li, X.; Li, M.-Y.; Yang, Y. High Performance ZnO Quantum Dot (QD)/ Magnetron Sputtered ZnO Homojunction Ultraviolet Photodetectors. Appl. Surf. Sci. 2022, 582, 152352. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Zeng, M.; Fu, L. Space-Confined Growth of Metal Halide Perovskite Crystal Films. Nano Res. 2020, 14, 1609–1624. [Google Scholar] [CrossRef]
- Huang, F.; Siffalovic, P.; Li, B.; Yang, S.; Zhang, L.; Nadazdy, P.; Cao, G.; Tian, J. Controlled Crystallinity and Morphologies of 2D Ruddlesden-Popper Perovskite Films Grown without Anti-Solvent for Solar Cells. Chem. Eng. J. 2020, 394, 124959. [Google Scholar] [CrossRef]
- Jung, M.H. Exploration of Two-Dimensional Perovskites Incorporating Methylammonium for High Performance Solar Cells. CrystEngComm 2021, 23, 1181–1200. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Gupta, V.; Yadav, T.P.; Gupta, V.; Singh, S.P. Green Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Quantum Dots Using Eclipta Alba. Mater. Chem. Phys. 2018, 203, 40–48. [Google Scholar] [CrossRef]
- Yang, S.; Niu, W.; Wang, A.-L.; Fan, Z.; Chen, B.; Tan, C.; Lu, Q.; Zhang, H. Ultrathin Two-Dimensional Organic-Inorganic Hybrid Perovskite Nanosheets with Bright, Tunable Photoluminescence and High Stability. Angew. Chem. Int. Ed. 2017, 56, 4252–4255. [Google Scholar] [CrossRef]
- Lan, J.; Lv, J.; Feng, J. Identification of Chrome Pigments in Paints with Fourier Transform Infrared Spectroscopy (FTIR), Confocal Raman Microscopy, and Scanning Electron Microscope-Energy Dispersive Spectrometer. Environ. Forensics 2013, 14, 81–86. [Google Scholar] [CrossRef]
- Sánchez, J.D.G.; Messina, S.; Álvarez, J.C.; Nair, P.K. Optical Absorption and Light-Generated Current Density in Chemically Deposited Antimony Sulfide Selenide Thin Films Used for Solar Cell Development. J. Mater. Sci. Mater. Electron. 2022, 33, 12026–12038. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, Y.; Paek, S.; Gao, X.-X.; Li, X.; Nazeeruddin, M.K. Enhanced Stability of α-Phase FAPbI3 Perovskite Solar Cells by Insertion of 2D (PEA)2PbI4 Nanosheets. J. Mater. Chem. A 2020, 8, 8058–8064. [Google Scholar] [CrossRef]
- Adhikari, N.; Dubey, A.; Gaml, E.A.; Vaagensmith, B.; Reza, K.M.; Mabrouk, S.A.A.; Gu, S.; Zai, J.; Qian, X.; Qiao, Q. Crystallization of a Perovskite Film for Higher Performance Solar Cells by Controlling Water Concentration in Methyl Ammonium Iodide Precursor Solution. Nanoscale 2016, 8, 2693–2703. [Google Scholar] [CrossRef]
- Tu, Y.; Xu, Y.; Li, J.; Hao, Q.; Liu, X.; Qi, D.; Bao, C.; He, T.; Gao, F.; Zhang, W. Ultrathin Single-Crystalline 2D Perovskite Photoconductor for High-Performance Narrowband and Wide Linear Dynamic Range Photodetection. Small 2020, 16, 2005626. [Google Scholar] [CrossRef]
- Song, J.; Fang, T.; Li, J.; Xu, L.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Organic–Inorganic Hybrid Passivation Enables Perovskite QLEDs with an EQE of 16.48%. Adv. Mater. 2018, 30, 1805409. [Google Scholar] [CrossRef]
- Chen, H.; Guo, A.; Zhu, J.; Cheng, L.; Wang, Q. Tunable Photoluminescence of CsPbBr3 Perovskite Quantum Dots for Their Physical Research. Appl. Surf. Sci. 2019, 465, 656–664. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, K.; Yang, F.; Cong, H.; Wang, N.; Zheng, J.; Zuo, Y.; Li, C.; Cheng, B.; Wang, Q. Insight into the Effect of Ligand-Exchange on Colloidal CsPbBr3 Perovskite Quantum Dot/Mesoporous-TiO2 Composite-Based Photodetectors: Much Faster Electron Injection. J. Mater. Chem. C 2017, 5, 6224–6233. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Liu, D.; Luo, J. Neutral and Defect-Induced Exciton Annihilation in Defective Monolayer WS2. Nanoscale 2019, 11, 7913–7920. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Xu, H.; Liu, W.; Zhang, C.; Wang, C.; Wang, Z.; Ma, J.; Liu, Y. Color-Tunable ZnO/GaN Heterojunction LEDs Achieved by Coupling with Ag Nanowire Surface Plasmons. ACS Appl. Mater. Interfaces 2018, 10, 15812–15819. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Li, C.; Wang, Y.; Deng, Z. Graded Interface Engineering of 3D/2D Halide Perovskite Solar Cells through Ultrathin (PEA)2PbI4 Nanosheets. Chin. Chem. Lett. 2021, 32, 2259–2262. [Google Scholar] [CrossRef]
- Lee, S.-W.; Cha, S.-H.; Choi, K.-J.; Kang, B.-H.; Lee, J.-S.; Kim, S.-W.; Kim, J.-S.; Jeong, H.-M.; Gopalan, S.-A.; Kwon, D.-H.; et al. Low Dark-Current, High Current-Gain of PVK/ZnO Nanoparticles Composite-Based UV Photodetector by PN-Heterojunction Control. Sensors 2016, 16, 74. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Guo, X.; Wang, W.; Zhang, Y.; Xu, S.; Lien, D.H.; Wang, Z.L. Enhancing Sensitivity of a Single ZnO Micro-/Nanowire Photodetector by Piezo-Phototronic Effect. ACS Nano 2010, 4, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tong, M.; Xia, Y.; Cai, W.; Moon, J.S.; Cao, Y.; Yu, G.; Shieh, C.-L.; Nilsson, B.; Heeger, A.J. High-Detectivity Polymer Photodetectors with Spectral Response from 300 nm to 1450 nm. Science 2009, 325, 1665–1667. [Google Scholar] [CrossRef]
- Xiao, H.; Liang, T.; Xu, M. Growth of Ultraflat PbI2 Nanoflakes by Solvent Evaporation Suppression for High-Performance UV Photodetectors. Small 2019, 15, 1901767. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Shao, Y.; Yuan, Y.; Xiao, Z.; Wang, C.; Gao, Y.; Huang, J. Understanding the Formation and Evolution of Interdiffusion Grown Organolead Halide Perovskite Thin Films by Thermal Annealing. J. Mater. Chem. A 2014, 2, 18508–18514. [Google Scholar] [CrossRef]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Duan, H.-S.; Yang, W.; Zhou, H.; Yang, Y. Benign Solutions and Innovative Sequential Annealing Processes for High Performance Cu2ZnSn(Se,S)4Photovoltaics. Adv. Energy Mater. 2013, 4, 1301287. [Google Scholar] [CrossRef]
- Yu, J.C.; Kim, D.W.; Kim, D.B.; Jung, E.D.; Park, J.H.; Lee, A.-Y.; Lee, B.R.; Di Nuzzo, D.; Friend, R.H.; Song, M.H. Improving the Stability and Performance of Perovskite Light-Emitting Diodes by Thermal Annealing Treatment. Adv. Mater. 2016, 28, 6906–6913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataprasad Bhat, S.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. Photoluminescence and Photoconducting Properties of ZnO Nanoparticles. Solid State Commun. 2009, 149, 510–514. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Improved Surface-Enhanced Raman Scattering Properties of TiO2Nanoparticles by Zn Dopant. J. Raman Spectrosc. 2009, 41, 721–726. [Google Scholar] [CrossRef]
- Cao, F.; Tian, W.; Gu, B.; Ma, Y.; Lu, H.; Li, L. High-Performance UV–Vis Photodetectors Based on Electrospun ZnO Nanofiber-Solution Processed Perovskite Hybrid Structures. Nano Res. 2017, 10, 2244–2256. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving All-Inorganic Perovskite Photodetectors by Preferred Orientation and Plasmonic Effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef]
- Han, S.; Wang, P.; Zhang, J.; Liu, X.; Sun, Z.; Huang, X.; Li, L.; Ji, C.; Zhang, W.; Teng, B.; et al. Exploring a Polar Two-Dimensional Multi-Layered Hybrid Perovskite of (C5H11NH3)2(CH3NH3)Pb2I7 for Ultrafast-Responding Photodetection. Laser Photonics Rev. 2018, 12, 1800060. [Google Scholar] [CrossRef]
- Maculan, G.; Sheikh, A.D.; Abdelhady, A.L.; Saidaminov, M.I.; Haque, M.A.; Murali, B.; Alarousu, E.; Mohammed, O.F.; Wu, T.; Bakr, O.M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Wang, H.; Shen, Z.; Shen, H.; Wang, S.; Ma, J.; Wang, J.; Luo, H.; Li, D. High-Performance Photodetectors Based on Lead-Free 2D Ruddlesden–Popper Perovskite/MoS2 Heterostructures. ACS Appl. Mater. Interfaces 2019, 11, 8419–8427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Xu, Z.; Yang, Z.; Liu, S. (Frank) 2D Perovskite Single Crystals with Suppressed Ion Migration for High-Performance Planar-Type Photodetectors. Small 2020, 16, 2003145. [Google Scholar] [CrossRef]
- Fang, Y.; Dong, Q.; Shao, Y.; Yuan, Y.; Huang, J. Highly Narrowband Perovskite Single-Crystal Photodetectors Enabled by Surface-Charge Recombination. Nat. Photonics 2015, 9, 679–686. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Weng, W.; Ji, C.; Liu, X.; Sun, Z.; Lin, W.; Hong, M.; Luo, J. Trilayered Lead Chloride Perovskite Ferroelectric Affording Self-Powered Visible-Blind Ultraviolet Photodetection with Large Zero-Bias Photocurrent. J. Am. Chem. Soc. 2019, 142, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Cheng, B.; Li, T.-Y.; Retamal, J.R.D.; Wei, T.-C.; Fu, H.-C.; Fang, X.; He, J.-H. Orthogonal Lithography for Halide Perovskite Optoelectronic Nanodevices. ACS Nano 2018, 13, 1168–1176. [Google Scholar] [CrossRef] [PubMed]







| PD1 | PD4 | |
|---|---|---|
| τ1 (ns) | 0.69 | 0.53 |
| τ2 (ns) | 3.10 | 3.27 |
| τ3 (ns) | 45.67 | - |
| B1 (%) | 53.52 | 84.82 |
| B2 (%) | 38.62 | 15.18 |
| B3 (%) | 7.87 | - |
| τavg (ns) | 5.16 | 0.94 |
| Photodetectors | Rs (μA/W) | D* (×109 Jones) | Ion/Ioff Ratio | τrise (ms) | τfall (ms) |
|---|---|---|---|---|---|
| PD1 | 14.21 | 1.32 | 78.3 | 95.8 | 106.8 |
| PD2 | 100.73 | 9.48 | 564 | 69.1 | 46.7 |
| PD3 | 142.40 | 13.50 | 809 | 126.7 | 53.0 |
| PD4 | 143.94 | 15.40 | 1040 | 53.3 | 87.0 |
| PD5 | 67.45 | 6.82 | 437 | 69.0 | 87.7 |
| PD6 | 36.99 | 3.93 | 265 | 42.9 | 89.0 |
| PD7 | 23.77 | 2.46 | 173 | 69.1 | 88.2 |
| PD8 | 39.81 | 1.73 | 48.6 | 53.3 | 86.6 |
| PD9 | 88.31 | 3.96 | 113 | 37.3 | 72.8 |
| PD10 | 130.88 | 9.87 | 473 | 53.4 | 82.1 |
| PD11 | 131.11 | 8.88 | 381 | 53.2 | 92.3 |
| Materials | Ioff (nA) | Bias (V) | Ion/Ioff Ratio | D* (×109 Jones) | Ref. |
|---|---|---|---|---|---|
| CH3NH3PbI3 | 1 | 1 | 3.05 | 900 | [51] |
| CsPbBr3 | 0.488 | 2 | 1.7 × 106 | 0.456 | [52] |
| (PA)2(MA)Pb2I7 | ~0.1 | 10 | >103 | 29.2 | [53] |
| MAPbI3 | >102 | 15 | 1100 | 12 | [54] |
| (PEA)2PbI4, MoS2 | ~103 | 3 | 500 | 8.09 | [55] |
| BDAPbI4 | >10−2 | 10 | / | ~1 | [56] |
| MAPbI3-xBrx | 4 | 4 | <10 | 20 | [57] |
| EA4Pb3Cl10 | / | 5 | ~104 | 3.06 | [58] |
| (PEA)2PbI4 | ~10−3 | 5 | 10.8 | 1070 | [59] |
| (PEA)2PbI4, ZnO | 4.37 × 10−3 | 10 | 1040 | 15.40 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, H.; Lu, H.; Wang, Y.; Wen, X.; Deng, S.; Li, M.-Y.; Liu, S.; Wang, C.; Li, X. High Performance 0D ZnO Quantum Dot/2D (PEA)2PbI4 Nanosheet Hybrid Photodetectors Fabricated via a Facile Antisolvent Method. Nanomaterials 2022, 12, 4217. https://doi.org/10.3390/nano12234217
Liu S, Li H, Lu H, Wang Y, Wen X, Deng S, Li M-Y, Liu S, Wang C, Li X. High Performance 0D ZnO Quantum Dot/2D (PEA)2PbI4 Nanosheet Hybrid Photodetectors Fabricated via a Facile Antisolvent Method. Nanomaterials. 2022; 12(23):4217. https://doi.org/10.3390/nano12234217
Chicago/Turabian StyleLiu, Shijie, Hao Li, Haifei Lu, Yanran Wang, Xiaoyan Wen, Shuo Deng, Ming-Yu Li, Sisi Liu, Cong Wang, and Xiao Li. 2022. "High Performance 0D ZnO Quantum Dot/2D (PEA)2PbI4 Nanosheet Hybrid Photodetectors Fabricated via a Facile Antisolvent Method" Nanomaterials 12, no. 23: 4217. https://doi.org/10.3390/nano12234217
APA StyleLiu, S., Li, H., Lu, H., Wang, Y., Wen, X., Deng, S., Li, M. -Y., Liu, S., Wang, C., & Li, X. (2022). High Performance 0D ZnO Quantum Dot/2D (PEA)2PbI4 Nanosheet Hybrid Photodetectors Fabricated via a Facile Antisolvent Method. Nanomaterials, 12(23), 4217. https://doi.org/10.3390/nano12234217