Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery
Abstract
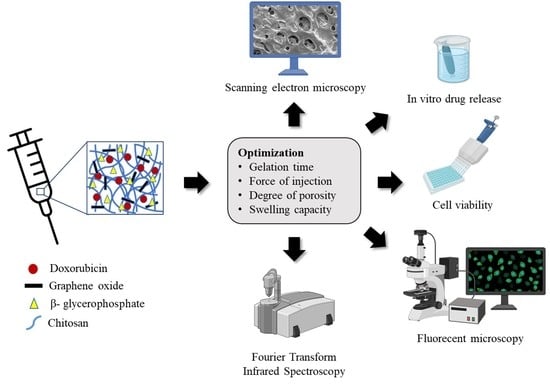
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of DOX-Loaded GO/CS/GP CH
2.4. Physicochemical Characterization of DOX-Loaded GO/CS/GP CH
2.4.1. Gelation Time
2.4.2. The Force Required for Injection
2.4.3. Degree of Porosity
2.4.4. Swelling Capacity
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6. Scanning Electron Microscopy
2.7. In Vitro Release Studies
2.8. Cell Culture
2.9. Cytotoxicity and IC50 Studies
2.10. Cellular Uptake
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation of DOX-Loaded GO/CS/GP CH
3.2. Optimization of DOX-Loaded GO/CS/GP CH
3.3. Effect on the Gelation Time
3.4. Effect on the Force Required for Injection
3.5. Effect on the Degree of Porosity
3.6. Effect on the Swelling Capacity
3.7. Selection of the Optimal DOX-Loaded GO/CS/GP CH Formulation
3.8. Physico-Chemical Characterization of DOX/opt GH
3.8.1. FT-IR Spectroscopy
3.8.2. Microstructure Analysis
3.8.3. In-Vitro Release Studies
3.9. Cytotoxicity and IC50 Studies
3.10. Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Soltani, M.; Hamedi, M.-H. Drug delivery to solid tumors with heterogeneous microvascular networks: Novel insights from image-based numerical modeling. Eur. J. Pharm. Sci. 2020, 151, 105399. [Google Scholar] [CrossRef] [PubMed]
- Botto, N.; Rogers, G. Nontraditional management of basal cell carcinoma. J. Drugs Dermatol. 2013, 12, 525–532. [Google Scholar] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Peyser, N.D.; Grandis, J.R. Integration of molecular targeted therapy with radiation in head and neck cancer. Pharmacol. Ther. 2014, 142, 88–98. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Haghshenas, B.; Eskandani, M.; Jahanban-Esfahlan, R.; Abbasi-Maleki, S.; Jaymand, M. Folate-conjugated thermal- and pH-responsive magnetic hydrogel as a drug delivery nano-system for “smart” chemo/hyperthermia therapy of solid tumors. Mater. Today Commun. 2022, 30, 103148. [Google Scholar] [CrossRef]
- Chua, C.Y.X.; Ho, J.; Demaria, S.; Ferrari, M.; Grattoni, A. Emerging technologies for local cancer treatment. Adv. Ther. 2020, 3, 2000027. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, S.; Milosevic, M.; Tannock, I.F.; Allen, C.; Jaffray, D.A. The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J. Control. Release 2015, 211, 163–170. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Hong, W.X.; Haebe, S.; Lee, A.S.; Westphalen, C.B.; Norton, J.A.; Jiang, W.; Levy, R. Intratumoral Immunotherapy for Early-stage Solid Tumors. Clin. Cancer Res. 2020, 26, 3091–3099. [Google Scholar] [CrossRef] [Green Version]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discov. Today 2021, 26, 2397–2405. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J.C. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Wang, T.; Gao, X.; Wan, Q.; Pei, X. Preparation and characterization of chitosan/β-glycerophosphate thermal-sensitive hydrogel reinforced by graphene oxide. Front. Chem. 2018, 6, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.B.; Patenaude, M.; Hoare, T. Injectable Superparamagnets: Highly Elastic and Degradable Poly(N-isopropylacrylamide)–Superparamagnetic Iron Oxide Nanoparticle (SPION) Composite Hydrogels. Biomacromolecules 2013, 14, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Moztarzadeh, F.; Haghighipour, N.; Ghazizadeh, L.; Baghbani, F.; Shokrgozar, M.A.; Allahyari, Z. Preparation and characterization of novel functionalized multiwalled carbon nanotubes/chitosan/β-Glycerophosphate scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Talaat, W.; Smriti Aryal, A.C.; Kawas, S.A.; Samsudin, A.B.R.; Kandile, N.G.; Harding, D.R.K.; Ghoneim, M.M.; Zeiada, W.; Jagal, J.; Aboelnaga, A.; et al. Nanoscale thermosensitive hydrogel scaffolds promote the chondrogenic differentiation of dental pulp stem and progenitor cells: A minimally invasive approach for cartilage regeneration. Int. J. Nanomed. 2020, 15, 7775–7789. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Feng, Z.; Yin, D.; Xu, Q.; Fan, L. Graphene oxide-based composite hydrogels with self-assembled macroporous structures. RSC Adv. 2016, 6, 3561–3570. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Zhou, J.; Li, X.; Liu, J.; Zeng, M. Mechanical enhancement of graphene oxide-filled chitosan-based composite hydrogels by multiple mechanisms. J. Mater. Sci. 2020, 55, 14690–14701. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Noroozi Pesyan, N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef]
- Al homsi, R.; Eltahir, S.; Jagal, J.; Ali Abdelkareem, M.; Ghoneim, M.M.; Rawas-Qalaji, M.M.; Greish, K.; Haider, M. Thermosensitive injectable graphene oxide/chitosan-based nanocomposite hydrogels for controlling the in vivo release of bupivacaine hydrochloride. Int. J. Pharm. 2022, 621, 121786. [Google Scholar] [CrossRef]
- Talaat, W.M.; Haider, M.; Al Kawas, S.; Kandil, N.G.; Harding, D.R.K. Chitosan-based thermosensitive hydrogel for controlled drug delivery to the temporomandibular joint. J. Craniofacial Surg. 2016, 27, 735–740. [Google Scholar] [CrossRef]
- Haider, M.; Hassan, M.A.; Ahmed, I.S.; Shamma, R. Thermogelling Platform for Baicalin Delivery for Versatile Biomedical Applications. Mol. Pharm. 2018, 15, 3478–3488. [Google Scholar] [CrossRef]
- Haider, M.; Elsayed, I.; Ahmed, I.S.; Fares, A.R. In situ-forming microparticles for controlled release of rivastigmine: In vitro optimization and in vivo evaluation. Pharmaceuticals 2021, 14, 66. [Google Scholar] [CrossRef]
- Haider, M.; Elsherbeny, A.; Jagal, J.; Hubatová-Vacková, A.; Saad Ahmed, I. Optimization and Evaluation of Poly(lactide-co-glycolide) Nanoparticles for Enhanced Cellular Uptake and Efficacy of Paclitaxel in the Treatment of Head and Neck Cancer. Pharmaceutics 2020, 12, 828. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Anuradha, D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018, 107, 908–917. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.G.B.; Nunes, C.S.; Rubira, A.F.; Muniz, E.C.; Fajardo, A.R. Effect of chitin nanowhiskers on mechanical and swelling properties of Gum Arabic hydrogels nanocomposites. Carbohydr. Polym. 2021, 266, 118116. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A hyaluronic acid/cyclodextrin based injectable hydrogel for local doxorubicin delivery to solid tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, Y. Weak bond-based injectable and stimuli responsive hydrogels for biomedical applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E.; Mohammed, Y. Quality by design: Development of the quality target product profile (QTPP) for semisolid topical products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef] [Green Version]
- Dalwadi, C.; Patel, G. Implementation of “Quality by Design (QbD)” Approach for the Development of 5-Fluorouracil Loaded Thermosensitive Hydrogel. Curr. Drug Deliv. 2016, 13, 512–527. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Kim, H.; Song, D.; Ngo, H.V.; Jin, G.; Park, C.; Park, J.B.; Lee, B.J. Modulation of the clinically accessible gelation time using glucono-d-lactone and pyridoxal 5′-phosphate for long-acting alginate in situ forming gel injectable. Carbohydr. Polym. 2021, 272, 118453. [Google Scholar] [CrossRef]
- Ganji, F.; Abdekhodaie, M.J.; Ramazani, A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J. Sol-Gel Sci. Technol. 2007, 42, 47–53. [Google Scholar] [CrossRef]
- Ma, D.; Lin, J.; Chen, Y.; Xue, W.; Zhang, L.M. In situ gelation and sustained release of an antitumor drug by graphene oxide nanosheets. Carbon 2012, 50, 3001–3007. [Google Scholar] [CrossRef]
- Jafari, Z.; Rad, A.S.; Baharfar, R.; Asghari, S.; Esfahani, M.R. Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate. J. Mol. Liq. 2020, 315, 113835. [Google Scholar] [CrossRef]
- Réeff, J.; Gaignaux, A.; Goole, J.; De Vriese, C.; Amighi, K. New sustained-release intraarticular gel formulations based on monolein for local treatment of arthritic diseases. Drug Dev. Ind. Pharm. 2013, 39, 1731–1741. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability evaluation: An open issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef]
- Park, M.R.; Seo, B.B.; Song, S.C. Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 2013, 34, 1327–1336. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Yasmeen, S.; Lo, M.K.; Bajracharya, S.; Roldo, M. Injectable scaffolds for bone regeneration. Langmuir 2014, 30, 12977–12985. [Google Scholar] [CrossRef] [Green Version]
- Ayyagari, A.V.; Mutyala, K.C.; Sumant, A.V. Towards developing robust solid lubricant operable in multifarious environments. Sci. Rep. 2020, 10, 15390. [Google Scholar] [CrossRef]
- Li, H.; Wu, C.W.; Wang, S.; Zhang, W. Mechanically strong poly (vinyl alcohol) hydrogel with macropores and high porosity. Mater. Lett. 2020, 266, 127504. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Rodríguez, V.; Pérez-Esteban, P.; Martín del Valle, E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef]
- Siboro, S.A.P.; Anugrah, D.S.B.; Ramesh, K.; Park, S.H.; Kim, H.R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhao, X.; Zhou, J.; Suo, Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids 2008, 56, 1779–1793. [Google Scholar] [CrossRef]
- Ebrahimi, R. The study of factors affecting the swelling of ultrasound-prepared hydrogel. Polym. Bull. 2019, 76, 1023–1039. [Google Scholar] [CrossRef]
- Van De Wetering, P.; Metters, A.T.; Schoenmakers, R.G.; Hubbell, J.A. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release 2005, 102, 619–627. [Google Scholar] [CrossRef]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef]
- Das, G.; Nicastri, A.; Coluccio, M.L.; Gentile, F.; Candeloro, P.; Cojoc, G.; Liberale, C.; De Angelis, F.; Di Fabrizio, E. FT-IR, Raman, RRS measurements and DFT calculation for doxorubicin. Microsc. Res. Tech. 2010, 73, 991–995. [Google Scholar] [CrossRef]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Adv. Polym. Technol. 2018, 37, 3665–3679. [Google Scholar] [CrossRef] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Li, J.; Li, J.; Fei, Y.; Dong, J.; Pan, W. Optimization of thermosensitive chitosan hydrogels for the sustained delivery of venlafaxine hydrochloride. Int. J. Pharm. 2013, 441, 482–490. [Google Scholar] [CrossRef]
- Mellati, A.; Hasanzadeh, E.; Gholipourmalekabadi, M.; Enderami, S.E. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater. Sci. Eng. C 2021, 131, 112489. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kim, J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, B.S.A.D.; de Assis, A.C.C.; Souza, N.M.; Ferreira, L.F.R.; Soriano, R.N.; Bilal, M.; Iqbal, H.M.N. Nanotherapeutic approach to tackle chemotherapeutic resistance of cancer stem cells. Life Sci. 2021, 279, 119667. [Google Scholar] [CrossRef]
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Consoli, V.; Alghamdi, M.A.; Hussain, Z.; Khoder, G.; Greish, K. Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1853. [Google Scholar] [CrossRef]






| Numerical Factors (Continuous) | Applied Levels | ||
|---|---|---|---|
| Low (−1) | High (+1) | ||
| X1 | GO concentration (%w/v) | 0 | 0.1 |
| X2 | CS concentration (%w/v) | 1.5 | 2 |
| Numerical Factor (Discrete) | Applied Levels | ||
| X3 | CS:GP ratio (v/v) | 2:1 | 3:1 |
| Responses (Units) | Optimization Goal | ||
| Y1 | Gelation time (min) | 3 min | |
| Y2 | Force required for injection (N) | Minimize | |
| Y3 | Degree of porosity (%) | Minimize | |
| Y4 | Swelling capacity (%) | Maximize | |
| Experimental Run | X1 (% w/v) | X2 (%w/v) | X3 (Ratio v/v) | Y1 (min) | Y2 (N) | Y3 (%) | Y4 (%) |
|---|---|---|---|---|---|---|---|
| F1 | 0.1 | 2 | 2:1 | 1.36 ± 0.15 | 5.69 ± 0.33 | 99.77 ± 11.23 | 129.11 ± 12.11 |
| F2 | 0.1 | 1.75 | 3:1 | 2.87 ± 0.11 | 6.19 ± 0.14 | 112.74 ± 9.85 | 294.56 ± 15.69 |
| F3 | 0.05 | 1.75 | 2:1 | 1.62 ± 0.42 | 5.96 ± 0.13 | 63.77 ± 7.43 | 96.84 ± 12.60 |
| F4 | 0 | 1.5 | 2:1 | 32.67 ± 0.57 | 4.84 ± 0.30 | 78.47 ± 7.77 | 47.46 ± 6.67 |
| F5 | 0.1 | 1.5 | 2:1 | 4.61 ± 0.11 | 4.26 ± 0.22 | 139.06 ± 8.43 | 46.55 ± 5.12 |
| F6 | 0.1 | 1.75 | 3:1 | 2.98 ± 0.39 | 6.02 ± 0.25 | 106.98 ± 11.59 | 301.38 ± 24.50 |
| F7 | 0.1 | 1.5 | 2:1 | 4.53 ± 0.06 | 4.07 ± 0.26 | 144.95 ± 7.30 | 41.27 ± 4.53 |
| F8 | 0.05 | 2 | 2:1 | 0.30 ± 0.03 | 6.40 ± 0.16 | 82.74 ± 9.81 | 127.42 ± 12.15 |
| F9 | 0 | 1.5 | 2:1 | 36.67 ± 0.57 | 4.84 ± 0.30 | 78.47 ± 7.77 | 47.46 ± 6.67 |
| F10 | 0.05 | 1.75 | 3:1 | 0.23 ± 0.02 | 5.91 ± 0.33 | 62.87 ± 16.73 | 317.46 ± 30.77 |
| F11 | 0.05 | 1.5 | 3:1 | 16.51 ± 0.34 | 2.43 ± 0.09 | 246.12 ± 31.02 | 129.22 ± 12.45 |
| F12 | 0.05 | 1.5 | 2:1 | 9.06 ± 1.29 | 5.82 ± 0.67 | 79.32 ± 9.27 | 106.33 ± 12.53 |
| F13 | 0 | 1.75 | 3:1 | 3.70 ± 1.30 | 7.01 ± 0.36 | 106.04 ± 10.59 | 189.02 ± 14.76 |
| F14 | 0.05 | 1.5 | 3:1 | 16.36 ± 0.23 | 2.52 ± 0.10 | 251.44 ± 21.91 | 133.87 ± 14.98 |
| F15 | 0.1 | 2 | 2:1 | 1.24 ± 0.12 | 5.82 ± 0.67 | 101.98 ± 12.66 | 121.36 ± 17.61 |
| F16 | 0 | 2 | 2:1 | 0.51 ± 0.08 | 7.94 ± 0.48 | 101.91 ± 12.95 | 86.29 ± 14.98 |
| F17 | 0 | 2 | 3:1 | 0.80 ± 0.39 | 10.10 ± 0.51 | 73.00 ± 12.52 | 170.83 ± 20.60 |
| F18 | 0.05 | 2 | 3:1 | 0.17 ± 0.02 | 7.74 ± 0.62 | 79.25 ± 6.89 | 335.66 ± 19.52 |
| F19 | 0 | 1.75 | 2:1 | 2.42 ± 0.18 | 6.12 ± 0.27 | 107.53 ± 16.12 | 49.58 ± 7.02 |
| QTPP Element | Target | Justification | |
|---|---|---|---|
| Dosage form | Injectable hydrogel | In situ drug delivery with long residence time | |
| Dosage design | Hydrophilic matrix | Sustain drug delivery with maximum biocompatibility and biodegradability | |
| Route of administration | Intratumoral | Site-specific delivery and minimal systemic toxicity | |
| Dosage product quality attributes | Physical attributes | Appearance | Sol-to-gel transition at body temperature for drug entrapment and controlled drug release |
| Performance attributes | Gelation time | Optimal for proper handling and controlling the drug release | |
| Force for injection | Ease of injection into the solid tumor | ||
| Porosity and swelling capacity | Control water uptake and drug release | ||
| Chemical attributes | Identification | Study possible chemical interactions between the ingredients of the formulation | |
| Response | Model Equation (p-Value) | R2 | Adj-R2 | Pred-R2 | Adequate Precision | Significant Terms |
|---|---|---|---|---|---|---|
| Gelation time (min) | Quadratic (p = 0.0014) | 0.947 | 0.912 | 0.845 | 16.98 | X1 (p = 0.002) X2 (p < 0.0001) X1X2 (p < 0.0001) X1X3 (p = 0.0236) X12 (p = 0.0342) X22 (p = 0.0003) |
| Force for injection (N) | 2FI (p = 0.001) | 0.903 | 0.875 | 0.799 | 20.533 | X1 (p = 0.0078) X2 (p < 0.0001) X2X3 (p < 0.0001) |
| Degree of porosity (%) | Quadratic (p = 0.0344) | 0.863 | 0.795 | 0.687 | 12.193 | X2 (p < 0.0001) X3 (p = 0.0064) X2X3 (p < 0.0001) X22 (p = 0.0061) |
| Swelling capacity (%) | Reduced Quadratic (p = 0.0384) | 0.922 | 0.883 | 0.771 | 14.847 | X1 (p = 0.001) X2 (p = 0.0001) X3 (p < 0.0001) X1X3 (p = 0.0043) X2X3 (p = 0.0124) |
| Formulation Variables | Values | Responses | Predicted Values | Observed Values |
|---|---|---|---|---|
| X1 | 0.1% | Y1 | 3 min | 1.18 ± 0.25 min |
| X2 | 1.7% | Y2 | 4.79 N | 4.03 ± 0.32 N |
| X3 | 3:1 | Y3 | 135.71% | 141.32 ± 54.89% |
| Y4 | 282.71% | 311.31 ± 67.55% |
| Cell Type | DOX/opt CH | Free DOX Solution | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| MCF 7 | 19.24 ± 1.22 | 6.47 ± 0.51 | 2.50 ± 0.19 | 1.64 ± 0.09 | 1.52 ± 0.09 | 1.48 ± 0.11 |
| MDB-MB-231 | 9.06 ± 0.78 | 6.13 ± 0.62 | 2.55 ± 0.17 | 2.33 ± 0.14 | 1.67 ± 0.14 | 1.53 ± 0.12 |
| FaDu | 14.67 ± 0.91 | 6.78 ± 0.43 | 3.03 ± 0.24 | 2.75 ± 0.18 | 1.21 ± 0.06 | 1.08 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltahir, S.; Al homsi, R.; Jagal, J.; Ahmed, I.S.; Haider, M. Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery. Nanomaterials 2022, 12, 4261. https://doi.org/10.3390/nano12234261
Eltahir S, Al homsi R, Jagal J, Ahmed IS, Haider M. Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery. Nanomaterials. 2022; 12(23):4261. https://doi.org/10.3390/nano12234261
Chicago/Turabian StyleEltahir, Safaa, Reem Al homsi, Jayalakshmi Jagal, Iman Saad Ahmed, and Mohamed Haider. 2022. "Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery" Nanomaterials 12, no. 23: 4261. https://doi.org/10.3390/nano12234261
APA StyleEltahir, S., Al homsi, R., Jagal, J., Ahmed, I. S., & Haider, M. (2022). Graphene Oxide/Chitosan Injectable Composite Hydrogel for Controlled Release of Doxorubicin: An Approach for Enhanced Intratumoral Delivery. Nanomaterials, 12(23), 4261. https://doi.org/10.3390/nano12234261