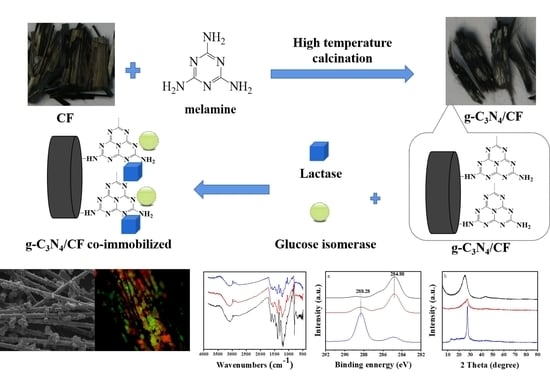
Co-Immobilization of Lactase and Glucose Isomerase on the Novel g-C3N4/CF Composite Carrier for Lactulose Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of g-C3N4/CF Composite Carriers
2.3. Co-Immobilization and SDS-PAGE Analysis of Lactase and Glucose Isomerase
2.4. Selection of the Composite Carrier with Enzyme Activity Ratio and the Kinetics of Co-Immobilized Enzymes
2.5. Enzyme Activity Assays



2.6. Characterization of Structure and Properties of Immobilized Enzyme with the g-C3N4/CF Composite Carriers
2.7. Assay of Co-Immobilized Enzyme Activity
2.7.1. Effect of Temperature and pH on Enzymes’ Activities
2.7.2. Storage Stability of Co-Immobilized Enzymes
2.7.3. Operational Stability of Co-Immobilized Enzymes
3. Results and Discussion
3.1. Structural Performance Characterization of the g-C3N4/CF Composite Carrier
3.2. The Apparent Morphology of the Co-Immobilized Enzyme and Its Distribution on the g-C3N4/CF
3.3. Enzymatic Properties Analysis of the g-C3N4/CF Co-Immobilized Double Enzyme
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simsek, E.B.; Saloglu, D. Exploring the structural and catalytic features of lipase enzymes immobilized on g-C3N4: A novel platform for biocatalytic and photocatalytic reactions. J. Mol. Liq. 2021, 337, 116612. [Google Scholar] [CrossRef]
- Ahmad, R.; Shanahan, J.; Rizaldo, S.; Kissel, D.S.; Stone, K.L. Co-immobilization of an Enzyme System on a Metal-Organic Framework to Produce a More Effective Biocatalyst. Catalysts 2020, 10, 499. [Google Scholar] [CrossRef]
- Long, J.; Pan, T.; Xie, Z.; Xu, X.; Jin, Z. Co-immobilization of β-fructofuranosidase and glucose oxidase improves the stability of Bi-enzymes and the production of lactosucrose. LWT 2020, 128, 109460. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Multi-faceted strategy based on enzyme immobilization with reactant adsorption and membrane technology for biocatalytic removal of pollutants: A critical review. Biotechnol. Adv. 2019, 37, 107401. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ren, D.; Jiang, S.; Yu, H.; Cheng, Y.; Zhang, S.; Zhang, X.; Chen, W. The study of laccase immobilization optimization and stability improvement on CTAB-KOH modified biochar. BMC Biotechnol. 2021, 21, 74. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Li, M.; Wang, X.; Rao, D.; Bai, X.; Shi, K.; Xu, H.; Hou, S.; Yao, H. Co-immobilized bienzyme of horseradish peroxidase and glucose oxidase on dopamine-modified cellulose–chitosan composite beads as a high-efficiency biocatalyst for degradation of acridine. RSC Adv. 2022, 12, 23006–23016. [Google Scholar] [CrossRef]
- Tentori, F.; Bavaro, T.; Brenna, E.; Colombo, D.; Monti, D.; Semproli, R.; Ubiali, D. Immobilization of Old Yellow Enzymes via Covalent or Coordination Bonds. Catalysts 2020, 10, 260. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yin, Z.; Cui, X.; Yang, L. Capillary electrophoresis-integrated immobilized enzyme microreactor with graphene oxide as support: Immobilization of negatively charged L-lactate dehydrogenase via hydrophobic interactions. Electrophoresis 2019, 41, 175–182. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Feng, Q.; Xu, T.; Wei, A.; Fong, H. Electrospun blend nanofiber membrane consisting of polyurethane, amidoxime polyarcylonitrile, and β-cyclodextrin as high-performance carrier/support for efficient and reusable immobilization of laccase. Chem. Eng. J. 2018, 331, 517–526. [Google Scholar] [CrossRef]
- Wang, L.; Sun, P.; Yang, Y.; Qiao, H.; Tian, H.; Wu, D.; Yang, S.; Yuan, Q.; Wang, J. Preparation of ZIF@ADH/NAD-MSN/LDH Core Shell Nanocomposites for the Enhancement of Coenzyme Catalyzed Double Enzyme Cascade. Nanomaterials 2021, 11, 2171. [Google Scholar] [CrossRef] [PubMed]
- Sulman, A.M.; Matveeva, V.G.; Bronstein, L.M. Cellulase Immobilization on Nanostructured Supports for Biomass Waste Processing. Nanomaterials 2022, 12, 3796. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.B.; Mulchandani, K.H.; Singhal, R.S. Immobilization of enzymes on iron oxide magnetic nanoparticles: Synthesis, characterization, kinetics and thermodynamics. Methods Enzymol. 2019, 630, 39–79. [Google Scholar] [CrossRef]
- Homouz, D.; Stagg, L.; Wittung-Stafshede, P.; Cheung, M.S. Macromolecular Crowding Modulates Folding Mechanism of α/β Protein Apoflavodoxin. Biophys. J. 2009, 96, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Chouyyok, W.; Panpranot, J.; Thanachayanant, C.; Prichanont, S. Effects of pH and pore characters of mesoporous silicas on horseradish peroxidase immobilization. J. Mol. Catal. B Enzym. 2009, 56, 246–252. [Google Scholar] [CrossRef]
- Romanovskaya, I.I.; Bondarenko, G.I.; Davidenko, T.I. Immobilization of Penicillium solitum lipase on the carbon fiber material “Dnepr-MN”. Pharm. Chem. J. 2008, 42, 360–362. [Google Scholar] [CrossRef]
- Garcia, L.F.; Siqueira, A.C.R.; Lobón, G.S.; Marcuzzo, J.S.; Pessela, B.C.; Mendez, E.; Garcia, T.A.; Gil, E.D.S. Bio-electro oxidation of indigo carmine by using microporous activated carbon fiber felt as anode and bioreactor support. Chemosphere 2017, 186, 519–526. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, S.; Bae, T.-S.; Lee, Y.-S. Enzyme biosensor based on an N-doped activated carbon fiber electrode prepared by a thermal solid-state reaction. Sens. Actuators B Chem. 2014, 197, 20–27. [Google Scholar] [CrossRef]
- Wang, L.; Jia, F.; Wu, D.; Wei, Q.; Liang, Y.; Hu, Y.; Li, R.; Yu, G.; Yuan, Q.; Wang, J. In-situ growth of graphene on carbon fibers for enhanced cell immobilization and xylitol fermentation. Appl. Surf. Sci. 2020, 527, 146793. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, G.; Bao, Y. Carbon nanotubes/carbon fiber hybrid material: A super support material for sludge biofilms. Environ. Technol. 2017, 39, 2105–2116. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, F. Enhanced visible light photocatalytic H2 production activity of g-C3N4 via carbon fiber. Appl. Surf. Sci. 2015, 358, 287–295. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Han, J.; Wang, L.; Zhang, W.; Dong, H.; Li, C.; Wang, Y. Photocatalyst/enzyme heterojunction fabricated for high-efficiency photoenzyme synergic catalytic degrading Bisphenol A in water. Chem. Eng. J. 2019, 385, 123764. [Google Scholar] [CrossRef]
- Tian, K.; Liu, H.; Dong, Y.; Chu, X.; Wang, S. Amperometric detection of glucose based on immobilizing glucose oxidase on g-C3N4 nanosheets. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123808. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, D.; Du, X. Ratiometric electrochemiluminescence sensing platform for sensitive glucose detection based on in situ generation and conversion of coreactants. Sens. Actuators B Chem. 2017, 251, 256–263. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, T.; Xu, P.; Zhang, L.; Liu, J.; Chen, Z. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl. Catal. B Environ. 2017, 219, 425–431. [Google Scholar] [CrossRef]
- Ma, T.; Bai, J.; Li, C. Facile synthesis of g-C 3 N 4 wrapping on one-dimensional carbon fiber as a composite photocatalyst to degrade organic pollutants. Vacuum 2017, 145, 47–54. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y.; et al. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2018, 158, 259–268. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Carballares, D.; Arana-Peña, S.; Siar, E.-H.; Braham, S.A.; Fernandez-Lafuente, R. Advantages of Supports Activated with Divinyl Sulfone in Enzyme Coimmobilization: Possibility of Multipoint Covalent Immobilization of the Most Stable Enzyme and Immobilization via Ion Exchange of the Least Stable Enzyme. ACS Sustain. Chem. Eng. 2021, 9, 7508–7518. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2020, 51, 107584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Hübner, R.; Tan, D.; Löffler, M.; Facsko, S.; Zhang, E.; Ge, Y.; Qi, Z.; Wu, C. Enzymes Immobilized on Carbon Nitride (C3N4) Cooperating with Metal Nanoparticles for Cascade Catalysis. Adv. Mater. Interfaces 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Bilal, M.; Hussain, N.; Américo-Pinheiro, J.H.P.; Almulaiky, Y.Q.; Iqbal, H.M. Multi-enzyme co-immobilized nano-assemblies: Bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges. Int. J. Biol. Macromol. 2021, 186, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hua, S.; Zhang, L. Co-immobilization of cellulase and glucose oxidase on graphene oxide by covalent bonds: A biocatalytic system for one-pot conversion of gluconic acid from carboxymethyl cellulose. J. Chem. Technol. Biotechnol. 2019, 95, 1116–1125. [Google Scholar] [CrossRef]
- Ubilla, C.; Ramírez, N.; Valdivia, F.; Vera, C.F.V.; Illanes, A.; Guerrero, C. Synthesis of Lactulose in Continuous Stirred Tank Reactor With β-Galactosidase of Apergillus oryzae Immobilized in Monofunctional Glyoxyl Agarose Support. Front. Bioeng. Biotechnol. 2020, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Lyu, X.; Hua, X.; Goddard, J.M.; Yang, R. Lactulose production from lactose isomerization by chemo-catalysts and enzymes: Current status and future perspectives. Biotechnol. Adv. 2022, 60, 108021. [Google Scholar] [CrossRef]
- Dong, L.; Yu, Z.; Zhao, R.; Peng, B.; Zhang, Y.; Wang, S. The effect of lactulose thermal degradation products on β-lactoglobulin: Linear-, loop-, and cross-link structural modifications and reduced digestibility. Food Chem. 2023, 403, 134333. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, M.; Hua, X.; Yao, C.; Yang, R. An innovative and sustainable adsorption-assisted isomerization strategy for the production and simultaneous purification of high-purity lactulose from lactose isomerization. Chem. Eng. J. 2020, 406, 126751. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Solingen, G.J.-V. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Karim, A.; Aider, M. Production of prebiotic lactulose through isomerisation of lactose as a part of integrated approach through whey and whey permeate complete valorisation: A review. Int. Dairy J. 2021, 126, 105249. [Google Scholar] [CrossRef]
- Karim, A.; Aider, M. Sustainable Valorization of Whey by Electroactivation Technology for In Situ Isomerization of Lactose into Lactulose: Comparison between Electroactivation and Chemical Processes at Equivalent Solution Alkalinity. ACS Omega 2020, 5, 8380–8392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, A.; Aider, M. Sustainable Electroisomerization of Lactose into Lactulose and Comparison with the Chemical Isomerization at Equivalent Solution Alkalinity. ACS Omega 2020, 5, 2318–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Albuquerque, T.L.; Gomes, S.D.L.; D’Almeida, A.P.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; Rocha, M.V.P. Immobilization of β-galactosidase in glutaraldehyde-chitosan and its application to the synthesis of lactulose using cheese whey as feedstock. Process. Biochem. 2018, 73, 65–73. [Google Scholar] [CrossRef]
- Guerrero, C.; Valdivia, F.; Ubilla, C.; Ramírez, N.; Gómez, M.; Aburto, C.; Vera, C.; Illanes, A. Continuous enzymatic synthesis of lactulose in packed-bed reactor with immobilized Aspergillus oryzae β-galactosidase. Bioresour. Technol. 2018, 278, 296–302. [Google Scholar] [CrossRef]
- Ramírez, N.; Ubilla, C.; Campos, J.; Valencia, F.; Aburto, C.; Vera, C.; Illanes, A.; Guerrero, C. Enzymatic production of lactulose by fed-batch and repeated fed-batch reactor. Bioresour. Technol. 2021, 341, 125769. [Google Scholar] [CrossRef]
- Ke, P.; Zeng, D.; Xu, K.; Cui, J.; Li, X.; Wang, G. Synthesis and characterization of a novel magnetic chitosan microsphere for lactase immobilization. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 606, 125522. [Google Scholar] [CrossRef]
- Ureta, M.M.; Martins, G.N.; Figueira, O.; Pires, P.F.; Castilho, P.C.; Gomez-Zavaglia, A. Recent advances in β-galactosidase and fructosyltransferase immobilization technology. Crit. Rev. Food Sci. Nutr. 2020, 61, 2659–2690. [Google Scholar] [CrossRef]
- Song, Y.S.; Lee, H.U.; Park, C.; Kim, S.W. Batch and continuous synthesis of lactulose from whey lactose by immobilized β-galactosidase. Food Chem. 2013, 136, 689–694. [Google Scholar] [CrossRef]
- Majore, K.; Ciprovica, I. Bioconversion of Lactose into Glucose–Galactose Syrup by Two-Stage Enzymatic Hydrolysis. Foods 2022, 11, 400. [Google Scholar] [CrossRef]
- Song, Y.-S.; Lee, H.-U.; Park, C.; Kim, S.-W. Optimization of lactulose synthesis from whey lactose by immobilized β-galactosidase and glucose isomerase. Carbohydr. Res. 2013, 369, 1–5. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Inanan, T. Cryogel disks for lactase immobilization and lactose-free milk production. LWT 2021, 154, 112608. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Sun, H.; Liu, H.; Mai, X.; Wang, X.; Wang, L.; Wang, N.; Huang, Y.; Guo, Z. Graphitic carbon nitride (g-C3N4) interfacially strengthened carbon fiber epoxy composites. Compos. Sci. Technol. 2018, 167, 515–521. [Google Scholar] [CrossRef]
- Li, C.; Yu, S.; Gu, L.; Han, J.; Dong, H.; Wang, Y.; Chen, G. A New Graphitic Carbon Nitride/Horseradish Peroxidase Hybrid Nano-Bio Artificial Catalytic System for Unselective Degradation of Persistent Phenolic Pollutants. Adv. Mater. Interfaces 2018, 5, 1801297. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Wang, L.; Liu, N.; Guo, Z.; Wu, D.; Chen, W.; Chang, Z.; Yuan, Q.; Hui, M.; Wang, J. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials 2016, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizi, N.S.; Shahzeydi, A.; Ghiaci, M.; Zhang, L. Photocatalytic degradation of cationic and anionic organic pollutants in water via Fe-g-C3N4/CF as a macroscopic photo-Fenton catalyst under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104219. [Google Scholar] [CrossRef]
- de Brito, A.R.; Tavares, I.M.D.C.; de Carvalho, M.S.; de Oliveira, A.J.; Salay, L.C.; Santos, A.S.; dos Anjos, P.N.M.; Oliveira, J.R.; Franco, M. Study of the interaction of the lactase enzyme immobilized in a carbon nanotube matrix for the development of the chemically modified carbon paste electrode. Surf. Interfaces 2020, 20, 100592. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2018, 355, 974–985. [Google Scholar] [CrossRef]
- Gennari, A.; Simon, R.; Sperotto, N.D.D.M.; Bizarro, C.V.; Basso, L.A.; Machado, P.; Benvenutti, E.V.; Renard, G.; Chies, J.M.; Volpato, G.; et al. Application of cellulosic materials as supports for single-step purification and immobilization of a recombinant β-galactosidase via cellulose-binding domain. Int. J. Biol. Macromol. 2022, 199, 307–317. [Google Scholar] [CrossRef]
- DE Carvalho, C.T.; Júnior, S.D.D.O.; Lima, W.B.D.B.; DE Medeiros, F.G.M.; Leitão, A.L.O.D.S.; Dantas, J.M.; DOS Santos, E.S.; DE Macêdo, G.R.; Júnior, F.C.d.S. Recovery of β-galactosidase produced by Kluyveromyces lactis by ion-exchange chromatography: Influence of pH and ionic strength parameters. An. Acad. Bras. Ciênc. 2022, 94, e20200752. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Isolation of Bacterial Strain with Xylanase and Xylose/Glucose Isomerase (GI) Activity and Whole Cell Immobilization for Improved Enzyme Production. Waste Biomass-Valoriz. 2020, 12, 833–845. [Google Scholar] [CrossRef]
- Xia, Y.; He, L.; Mao, J.; Fang, P.; Ma, X.; Wang, Z. Purification, characterization, and gene cloning of a new cold-adapted β-galactosidase from Erwinia sp. E602 isolated in northeast China. J. Dairy Sci. 2018, 101, 6946–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process. Biochem. 2019, 90, 66–80. [Google Scholar] [CrossRef]
- Keshta, B.E.; Gemeay, A.H.; Khamis, A.A. Impacts of horseradish peroxidase immobilization onto functionalized superparamagnetic iron oxide nanoparticles as a biocatalyst for dye degradation. Environ. Sci. Pollut. Res. 2021, 29, 6633–6645. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ming, H.; Li, L.; Li, M.; Gao, J.; Han, T.; Wang, Y. Fabrication of an in-situ co-immobilized enzyme in mesoporous silica for synthesizing GSH with ATP regeneration. Mol. Catal. 2020, 486, 110870. [Google Scholar] [CrossRef]
- Souza, C.J.; Garcia-Rojas, E.E.; Favaro-Trindade, C.S. Lactase (β-galactosidase) immobilization by complex formation: Impact of biopolymers on enzyme activity. Food Hydrocoll. 2018, 83, 88–96. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, Y.; Jiang, B.; Zhang, T.; Chen, J. Dual-enzyme co-immobilization for the one-pot production of glucose 6-phosphate from maltodextrin. Biochem. Eng. J. 2020, 161, 107654. [Google Scholar] [CrossRef]
- Mehnati-Najafabadi, V.; Taheri-Kafrani, A.; Bordbar, A.-K. Xylanase immobilization on modified superparamagnetic graphene oxide nanocomposite: Effect of PEGylation on activity and stability. Int. J. Biol. Macromol. 2018, 107, 418–425. [Google Scholar] [CrossRef]







| CF | g-C3N4/CF | |
|---|---|---|
| SBET (m2 g−1) | 0.61 ± 0.04 | 5.08 ± 0.04 |
| Microporous volume (cm3 g−1) | 1.91 × 10−4 ± 1.14 × 10−5 | 2.15 × 10−3 ± 1.39 × 10−5 |
| Mesoporous volume (cm3 g−1) | 1.76 × 10−3 ± 2.24 × 10−4 | 2.75 × 10−2 ± 1.23 × 10−3 |
| Total pore volume (cm3 g−1) | 1.95 × 10−3 ± 2.27 × 10−4 | 2.96 × 10−2 ± 1.23 × 10−3 |
| Average pore diameter (nm) | 12.87 ± 1.65 | 23.35 ± 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jiao, B.; Shen, Y.; Du, R.; Yuan, Q.; Wang, J. Co-Immobilization of Lactase and Glucose Isomerase on the Novel g-C3N4/CF Composite Carrier for Lactulose Production. Nanomaterials 2022, 12, 4290. https://doi.org/10.3390/nano12234290
Wang L, Jiao B, Shen Y, Du R, Yuan Q, Wang J. Co-Immobilization of Lactase and Glucose Isomerase on the Novel g-C3N4/CF Composite Carrier for Lactulose Production. Nanomaterials. 2022; 12(23):4290. https://doi.org/10.3390/nano12234290
Chicago/Turabian StyleWang, Le, Bingyu Jiao, Yan Shen, Rong Du, Qipeng Yuan, and Jinshui Wang. 2022. "Co-Immobilization of Lactase and Glucose Isomerase on the Novel g-C3N4/CF Composite Carrier for Lactulose Production" Nanomaterials 12, no. 23: 4290. https://doi.org/10.3390/nano12234290
APA StyleWang, L., Jiao, B., Shen, Y., Du, R., Yuan, Q., & Wang, J. (2022). Co-Immobilization of Lactase and Glucose Isomerase on the Novel g-C3N4/CF Composite Carrier for Lactulose Production. Nanomaterials, 12(23), 4290. https://doi.org/10.3390/nano12234290