Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterization
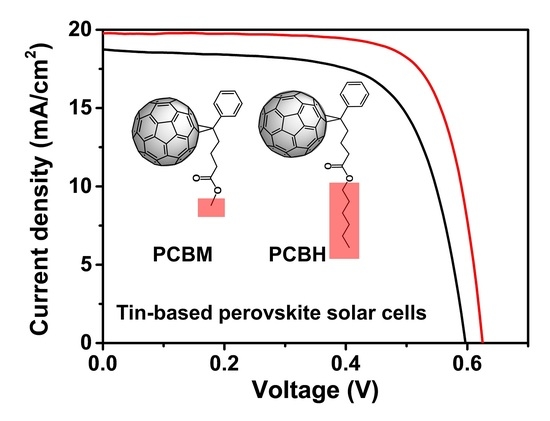
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, B.-B.; Chen, Z.; Zhu, Y.; Wang, Y.; Han, B.; Chen, G.; Zhang, X.; Du, Z.; He, Z. Heterogeneous 2D/3D Tin-Halides Perovskite Solar Cells with Certified Conversion Efficiency Breaking 14%. Adv. Mater. 2021, 33, 2102055. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Luo, X.; Lin, X.; Cui, D.; Wang, Y.; Segawa, H.; Zhang, Y.; Han, L. Lead-free tin perovskite solar cells. Joule 2021, 5, 863–886. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Zhou, Q.; Wei, Q.; Wei, M.; Jiang, L.; Wang, Z.; Peng, Z.; Wang, F.; Zang, Z.; et al. One-Step Synthesis of SnI2·(DMSO)x Adducts for High-Performance Tin Perovskite Solar Cells. J. Am. Chem. Soc. 2021, 143, 10970–10976. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, M.; Zhang, Y.; Ma, X.; Dong, J.; Lu, F.; Wang, J.; Wang, N.; Zhou, Y. Chemo-thermal surface dedoping for high-performance tin perovskite solar cells. Matter 2022. [Google Scholar] [CrossRef]
- Xu, L.; Feng, X.; Jia, W.; Lv, W.; Mei, A.; Zhou, Y.; Zhang, Q.; Chen, R.-F.; Huang, W. Recent Advances in Inverted Lead-free Tin-Based Perovskite Solar Cells and Challenges. Energy Environ. Sci. 2021, 14, 4292–4317. [Google Scholar] [CrossRef]
- Cao, J.; Yan, F. Recent progress in tin-based perovskite solar cells. Energy Environ. Sci. 2021, 14, 1286–1325. [Google Scholar] [CrossRef]
- Fang, Y.; Bi, C.; Wang, D.; Huang, J. The Functions of Fullerenes in Hybrid Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 782–794. [Google Scholar] [CrossRef]
- Deng, L.-L.; Xie, S.-Y.; Gao, F. Fullerene-Based Materials for Photovoltaic Applications: Toward Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells. Adv. Electron. Mater. 2018, 4, 1700435. [Google Scholar] [CrossRef] [Green Version]
- Castro, E.; Murillo, J.; Fernandez-Delgado, O.; Echegoyen, L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651. [Google Scholar] [CrossRef]
- Pascual, J.; Delgado, J.L.; Tena-Zaera, R. Physicochemical Phenomena and Application in Solar Cells of Perovskite:Fullerene Films. J. Phys. Chem. Lett. 2018, 9, 2893–2902. [Google Scholar] [CrossRef]
- Jia, L.; Chen, M.; Yang, S. Functionalization of fullerene materials toward applications in perovskite solar cells. Mater. Chem. Front. 2020, 4, 2256–2282. [Google Scholar] [CrossRef]
- Ran, C.; Gao, W.; Li, J.; Xi, J.; Li, L.; Dai, J.; Yang, Y.; Gao, X.; Dong, H.; Jiao, B.; et al. Conjugated Organic Cations Enable Efficient Self-Healing FASnI3 Solar Cells. Joule 2019, 3, 3072–3087. [Google Scholar] [CrossRef]
- Dai, S.-M.; Zhang, X.; Chen, W.-Y.; Li, X.; Tan, Z.A.; Li, C.; Deng, L.-L.; Zhan, X.-X.; Lin, M.-S.; Xing, Z.; et al. Formulation engineering for optimizing ternary electron acceptors exemplified by isomeric PC71BM in planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 18776–18782. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Xu, P.-Y.; Tian, H.-R.; Deng, L.-L.; Yao, Y.-R.; Chen, B.-W.; Xie, F.-F.; An, M.-W.; Yun, D.-Q.; et al. Crystallographic Understanding of Photoelectric Properties for C60 Derivatives Applicable as Electron Transporting Materials in Perovskite Solar Cells. Chem. Res. Chin. Univ. 2021, 38, 75–81. [Google Scholar] [CrossRef]
- Tian, C.; Castro, E.; Wang, T.; Betancourt-Solis, G.; Rodriguez, G.; Echegoyen, L. Improved Performance and Stability of Inverted Planar Perovskite Solar Cells Using Fulleropyrrolidine Layers. ACS Appl. Mater. Interfaces 2016, 8, 31426–31432. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Zhou, W.; Chen, M.; Li, B.; Jia, L.; Wang, M.; Yang, S. Pyridine-functionalized fullerene additive enabling coordination interactions with CH3NH3PbI3 perovskite towards highly efficient bulk heterojunction solar cells. J. Mater. Chem. A 2019, 7, 2754–2763. [Google Scholar] [CrossRef]
- Li, B.; Zhen, J.; Wan, Y.; Lei, X.; Jia, L.; Wu, X.; Zeng, H.; Chen, M.; Wang, G.-W.; Yang, S. Steering the electron transport properties of pyridine-functionalized fullerene derivatives in inverted perovskite solar cells: The nitrogen site matters. J. Mater. Chem. A 2020, 8, 3872–3881. [Google Scholar] [CrossRef]
- Collavini, S.; Kosta, I.; Völker, S.F.; Cabanero, G.; Grande, H.J.; Tena-Zaera, R.; Delgado, J.L. Efficient Regular Perovskite Solar Cells Based on Pristine [70] Fullerene as Electron-Selective Contact. Chemsuschem 2016, 9, 1263–1270. [Google Scholar] [CrossRef]
- Fernandez-Delgado, O.; Castro, E.; Ganivet, C.R.; Fosnacht, K.; Liu, F.; Mates, T.; Liu, Y.; Wu, X.; Echegoyen, L. Variation of Interfacial Interactions in PC61BM-like Electron-Transporting Compounds for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 34408–34415. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, W.; Luo, J.; Pellet, N.; Yi, C.; Li, X.; Zhao, X.; Dennis, T.J.S.; Li, X.; Wang, S.; et al. Isomer-Pure Bis-PCBM-Assisted Crystal Engineering of Perovskite Solar Cells Showing Excellent Efficiency and Stability. Adv. Mater. 2017, 29, 1606801. [Google Scholar] [CrossRef]
- Liang, Y.; Song, P.; Tian, H.; Tian, C.; Tian, W.; Nan, Z.; Cai, Y.; Yang, P.; Sun, C.; Chen, J.; et al. Lead Leakage Preventable Fullerene-Porphyrin Dyad for Efficient and Stable Perovskite Solar Cells. Adv. Funct. Mater. 2021, 2110139. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy 2016, 1, 16148. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wu, C.-G. Bulk heterojunction perovskite-PCBM solar cells with high fill factor. Nat. Photonics 2016, 10, 196–200. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, S.; Mei, A.; Rong, Y.; Hu, Y.; Du, K.; Duan, M.; Sheng, Y.; Jiang, P.; Xu, G.; et al. A Multifunctional Bis-Adduct Fullerene for Efficient Printable Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 10835–10841. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, S.; Wu, J.; Zhang, H.; Qin, M.; Lu, X.; Tu, Y.; Meng, Q.; Zhan, X. Fullerene derivative anchored SnO2 for high-performance perovskite solar cells. Energy Environ. Sci. 2018, 11, 3463–3471. [Google Scholar] [CrossRef]
- Tian, C.; Lin, K.; Lu, J.; Feng, W.; Song, P.; Xie, L.; Wei, Z. Interfacial Bridge Using a cis-Fulleropyrrolidine for Efficient Planar Perovskite Solar Cells with Enhanced Stability. Small Methods 2020, 4, 1900476. [Google Scholar] [CrossRef]
- Yu, W.; Sun, X.; Xiao, M.; Hou, T.; Liu, X.; Zheng, B.; Yu, H.; Zhang, M.; Huang, Y.; Hao, X. Recent advances on interface engineering of perovskite solar cells. Nano Res. 2022, 15, 85–103. [Google Scholar] [CrossRef]
- Li, S.-H.; Xing, Z.; Wu, B.-S.; Chen, Z.-C.; Yao, Y.-R.; Tian, H.-R.; Li, M.-F.; Yun, D.-Q.; Deng, L.-L.; Xie, S.-Y.; et al. Hybrid Fullerene-Based Electron Transport Layers Improving the Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 20733–20740. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Betancourt-Solis, G.; Nan, Z.; Liu, K.; Lin, K.; Lu, J.; Xie, L.; Echegoyen, L.; Wei, Z. Efficient and stable inverted perovskite solar cells enabled by inhibition of self-aggregation of fullerene electron-transporting compounds. Sci. Bull. 2021, 66, 339–346. [Google Scholar] [CrossRef]
- Troshin, P.A.; Hoppe, H.; Renz, J.; Egginger, M.; Mayorova, J.Y.; Goryochev, A.E.; Peregudov, A.S.; Lyubovskaya, R.N.; Gobsch, G.; Sariciftci, N.S.; et al. Material Solubility-Photovoltaic Performance Relationship in the Design of Novel Fullerene Derivatives for Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 779–788. [Google Scholar] [CrossRef]
- Tian, C.; Castro, E.; Betancourt-Solis, G.; Nan, Z.; Fernandez-Delgado, O.; Jankuru, S.; Echegoyen, L. Fullerene derivative with a branched alkyl chain exhibits enhanced charge extraction and stability in inverted planar perovskite solar cells. New J. Chem. 2018, 42, 2896–2902. [Google Scholar] [CrossRef]
- Pascual, J.; Nasti, G.; Aldamasy, M.H.; Smith, J.A.; Flatken, M.; Phung, N.; Di Girolamo, D.; Turren-Cruz, S.-H.; Li, M.; Dallmann, A.; et al. Origin of Sn(ii) oxidation in tin halide perovskites. Mater. Adv. 2020, 1, 1066–1070. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Wei, Q.; Li, H.; Shang, Y.; Zhou, W.; Wang, C.; Cheng, P.; Chen, Q.; Chen, L.; et al. Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design. Nat. Commun. 2020, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, F.; Li, S.H.; Chen, Z.C.; An, M.W.; Zheng, S.; Jen, A.K.Y.; Yang, S. Multifunctional Molecular Design of a New Fulleropyrrolidine Electron Transport Material Family Engenders High Performance of Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2107695. [Google Scholar] [CrossRef]
- Xu, G.; Xue, R.; Chen, W.; Zhang, J.; Zhang, M.; Chen, H.; Cui, C.; Li, H.; Li, Y.; Li, Y. New Strategy for Two-Step Sequential Deposition: Incorporation of Hydrophilic Fullerene in Second Precursor for High-Performance p-i-n Planar Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703054. [Google Scholar] [CrossRef]
- Tian, C.; Kochiss, K.; Castro, E.; Betancourt-Solis, G.; Han, H.; Echegoyen, L. A dimeric fullerene derivative for efficient inverted planar perovskite solar cells with improved stability. J. Mater. Chem. A 2017, 5, 7326–7332. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Hui, Y.; Wu, B.-S.; Chen, Z.-C.; Yun, D.-Q.; Deng, L.-L.; Zhang, M.-L.; Mao, B.-W.; Xie, S.-Y.; et al. Star-like hexakis[di(ethoxycarbonyl)methano]-C60 with higher electron mobility: An unexpected electron extractor interfaced in photovoltaic perovskites. Nano Energy 2020, 74, 104859. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ahmed, M.H.; Khan, M.I.; Miah, M.S.; Hossain, S. Recent Progress of Rare Earth Oxides for Sensor, Detector, and Electronic Device Applications: A Review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Yang, J.; Sheng, W.; Xiao, S.; Liu, G.; Lin, Z.; Tan, L.; Chen, Y. Directional Crystallization by Floating Self-Assembly for Efficient and Stable Tin-based Perovskite Solar Cells. Chem. Mater. 2021, 33, 4362–4372. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, S.; Ahmed, M.H.; Khan, M.I.; Haque, N.; Raihan, G.A. A Review on Optical Applications, Prospects, and Challenges of Rare-Earth Oxides. ACS Appl. Electron. Mater. 2021, 3, 3715–3746. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Rho, W.-Y.; Kohan, M.; Im, Y.H.; Mathur, S.; Hahn, Y.-B. Suppression of Sn2+/Sn4+ oxidation in tin-based perovskite solar cells with graphene-tin quantum dots composites in active layer. Nano Energy 2021, 90, 106495. [Google Scholar] [CrossRef]




| Fullerenes | λabs (nm) | Eg (eV) | LUMO (eV) | HOMO (eV) | |
|---|---|---|---|---|---|
| PCBH | 720 | 1.72 | −0.91 | −3.89 | −5.61 |
| PCBM | 718 | 1.73 | −0.90 | −3.90 | −5.63 |
| Type | Voc (V) | Jsc (mA/cm2) | Calculated Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PCBH | 0.63 | 19.77 | 19.60 | 73.96 | 9.21 |
| PCBM | 0.60 | 18.74 | 18.87 | 67.40 | 7.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Sun, C.; Chen, J.; Song, P.; Hou, E.; Xu, P.; Liang, Y.; Yang, P.; Luo, J.; Xie, L.; et al. Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells. Nanomaterials 2022, 12, 532. https://doi.org/10.3390/nano12030532
Tian C, Sun C, Chen J, Song P, Hou E, Xu P, Liang Y, Yang P, Luo J, Xie L, et al. Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells. Nanomaterials. 2022; 12(3):532. https://doi.org/10.3390/nano12030532
Chicago/Turabian StyleTian, Chengbo, Chao Sun, Jingfu Chen, Peiquan Song, Enlong Hou, Peng Xu, Yuming Liang, Panpan Yang, Jiefeng Luo, Liqiang Xie, and et al. 2022. "Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells" Nanomaterials 12, no. 3: 532. https://doi.org/10.3390/nano12030532
APA StyleTian, C., Sun, C., Chen, J., Song, P., Hou, E., Xu, P., Liang, Y., Yang, P., Luo, J., Xie, L., & Wei, Z. (2022). Fullerene Derivative with Flexible Alkyl Chain for Efficient Tin-Based Perovskite Solar Cells. Nanomaterials, 12(3), 532. https://doi.org/10.3390/nano12030532