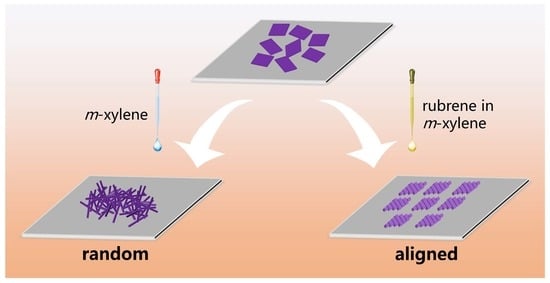
Rubrene-Directed Structural Transformation of Fullerene (C60) Microsheets to Nanorod Arrays with Enhanced Photoelectrochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Liao, Q.; Yao, J. Construction and Optoelectronic Properties of Organic One-Dimensional Nanostructures. Acc. Chem. Res. 2010, 43, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zheng, Q. Controlled Synthesis and Energy Applications of One-Dimensional Conducting Polymer Nanostructures: An Overview. Adv. Energy Mater. 2012, 2, 179–218. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Jiang, X.; Zhang, Z.; Wang, X.; Su, B.; Jiang, L. Positioning and Joining of Organic Single-Crystalline Wires. Nat. Commun. 2015, 6, 6737. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-Y.; Xu, H.; Wang, J.J.; Winters, S.; Motta, C.; Karademir, E.; Zhu, W.; Varrla, E.; Duesberg, G.S.; Sanvito, S.; et al. Vertical Single-Crystalline Organic Nanowires on Graphene: Solution-Phase Epitaxy and Optical Microcavities. Nano Lett. 2016, 16, 4754–4762. [Google Scholar] [CrossRef]
- Wang, Y.; Torres, J.A.; Stieg, A.Z.; Jiang, S.; Yeung, M.T.; Rubin, Y.; Chaudhuri, S.; Duan, X.; Kaner, R.B. Graphene-Assisted Solution Growth of Vertically Oriented Organic Semiconducting Single Crystals. ACS Nano 2015, 9, 9486–9496. [Google Scholar] [CrossRef]
- Deng, W.; Lv, Y.; Zhang, X.; Fang, X.; Lu, B.; Lu, Z.; Jie, J. High-Resolution Patterning of Organic Semiconductor Single Crystal Arrays for High-Integration Organic Field-Effect Transistors. Mater. Today 2020, 40, 82–90. [Google Scholar] [CrossRef]
- Tang, Q.; Li, H.; Song, Y.; Xu, W.; Hu, W.; Jiang, L.; Liu, Y.; Wang, X.; Zhu, D. In Situ Patterning of Organic Single-Crystalline Nanoribbons on a SiO2 Surface for the Fabrication of Various Architectures and High-Quality Transistors. Adv. Mater. 2006, 18, 3010–3014. [Google Scholar] [CrossRef]
- Zhang, X.; Jie, J.; Deng, W.; Shang, Q.; Wang, J.; Wang, H.; Chen, X.; Zhang, X. Alignment and Patterning of Ordered Small-Molecule Organic Semiconductor Micro-/Nanocrystals for Device Applications. Adv. Mater. 2016, 28, 2475–2503. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Dong, H.; Hu, W. Controlled Growth and Assembly of One-Dimensional Ordered Nanostructures of Organic Functional Materials. Soft Matter 2011, 7, 1615–1630. [Google Scholar] [CrossRef]
- Nam, S.; Jang, J.; Anthony, J.E.; Park, J.-J.; Park, C.E.; Kim, K. High-Performance Triethylsilylethynyl Anthradithiophene Transistors Prepared without Solvent Vapor Annealing: The Effects of Self-Assembly During Dip-Coating. ACS Appl. Mater. Interfaces 2013, 5, 2146–2154. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Kumatani, A.; Darmawan, P.; Minari, T.; Tsukagoshi, K. Large Plate-Like Organic Crystals from Direct Spin-Coating for Solution-Processed Field-Effect Transistor Arrays with High Uniformity. Org. Electron. 2012, 13, 264–272. [Google Scholar] [CrossRef]
- Thomas, A.; Goettmann, F.; Antonietti, M. Hard Templates for Soft Materials: Creating Nanostructured Organic Materials. Chem. Mater. 2008, 20, 738–755. [Google Scholar] [CrossRef]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Highly Ordered 1d Fullerene Crystals for Concurrent Control of Macroscopic Cellular Orientation and Differentiation toward Large-Scale Tissue Engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A High-Mobility Electron-Transporting Polymer for Printed Transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef]
- Wei, L.; Wu, Y.; Wang, L.; Fu, H.; Yao, J. Supramolecular Synthesis of Fullerene/Tetracene Hybrid Flowerlike Microstructures of Nanoplates Via the Charge-Transfer Interactions. J. Phys. Chem. C 2011, 115, 21629–21634. [Google Scholar] [CrossRef]
- Li, H.; Tee, B.C.K.; Cha, J.J.; Cui, Y.; Chung, J.W.; Lee, S.Y.; Bao, Z. High-Mobility Field-Effect Transistors from Large-Area Solution-Grown Aligned C60 Single Crystals. J. Am. Chem. Soc. 2012, 134, 2760–2765. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerenes: Three Dimensional Electron Acceptor Materials. Chem. Commun. 2000, 5, 321–327. [Google Scholar] [CrossRef]
- Miyazawa, K.; Kuwasaki, Y.; Obayashi, A.; Kuwabara, M. C60 Nanowhiskers Formed by the Liquid—Liquid Interfacial Precipitation Method. J. Mater. Res. 2002, 17, 83–88. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, W.; Skelton, P.; Yue, W.; Kinloch, I.A.; Windle, A.H.; Johnson, B.F.G. Crystal Structure and Growth Mechanism of Unusually Long Fullerene (C60) Nanowires. J. Am. Chem. Soc. 2008, 130, 2527–2534. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Park, J.E.; Chu, K.; Choi, H.C. Vertical Crystallization of C60 Nanowires by Solvent Vapor Annealing Process. ACS Nano 2013, 7, 9122–9128. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cuong, N.T.; Okada, S.; Xu, T.; Shen, W.; Lu, X.; Tsukagoshi, K. Solvent-Mediated Shape Engineering of Fullerene (C60) Polyhedral Microcrystals. Chem. Mater. 2018, 30, 7146–7153. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K. Size-Tunable Hexagonal Fullerene (C60) Nanosheets at the Liquid—Liquid Interface. J. Am. Chem. Soc. 2007, 129, 13816–13817. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Jiang, L.; Luo, H.; Xiao, S.; Fang, H.; Li, H.; Zhu, D.; Yu, D.; Xu, J.; et al. Imaging as-Grown [60] Fullerene Nanotubes by Template Technique. J. Am. Chem. Soc. 2002, 124, 13370–13371. [Google Scholar] [CrossRef]
- Guo, Y.G.; Li, C.J.; Wan, L.J.; Chen, D.M.; Wang, C.R.; Bai, C.L.; Wang, Y.G. Well-Defined Fullerene Nanowire Arrays. Adv. Funct. Mater. 2003, 13, 626–630. [Google Scholar] [CrossRef]
- Zheng, S.; Xiong, X.; Zheng, Z.; Xu, T.; Zhang, L.; Zhai, T.; Lu, X. Solution-Grown Large-Area C60 Single-Crystal Arrays as Organic Photodetectors. Carbon 2018, 126, 299–304. [Google Scholar] [CrossRef]
- Bairi, P.; Kumar, G.S.; Acharya, S.; Maji, S.; Ariga, K.; Shrestha, L.K. Vortex-Aligned Ordered Film of Crystalline Fullerene C70 Microtubes with Enhanced Photoluminescence and Photovoltaics Properties. J. Nanosci. Nanotechnol. 2020, 20, 2971–2978. [Google Scholar] [CrossRef]
- Guo, C.F.; Cao, S.; Zhang, J.; Tang, H.; Guo, S.; Tian, Y.; Liu, Q. Topotactic Transformations of Superstructures: From Thin Films to Two-Dimensional Networks to Nested Two-Dimensional Networks. J. Am. Chem. Soc. 2011, 133, 8211–8215. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, Y.; Hu, W.; Wong, W.-Y. Epitaxial Growth of Nanorod Meshes from Luminescent Organic Cocrystals Via Crystal Transformation. J. Am. Chem. Soc. 2020, 142, 7265–7269. [Google Scholar] [CrossRef]
- Li, L.; Sun, N.; Huang, Y.; Qin, Y.; Zhao, N.; Gao, J.; Li, M.; Zhou, H.; Qi, L. Topotactic Transformation of Single-Crystalline Precursor Discs into Disc-Like Bi2s3 Nanorod Networks. Adv. Funct. Mater. 2008, 18, 1194–1201. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, S.; Lai, Z.; Yao, X.; Zhao, Y.; Zhang, H.; Chen, H. Two-Dimensional C60 Nano-Meshes Via Crystal Transformation. Nanoscale 2019, 11, 8692–8698. [Google Scholar] [CrossRef]
- Sathish, M.; Miyazawa, K.i.; Hill, J.P.; Ariga, K. Solvent Engineering for Shape-Shifter Pure Fullerene (C60). J. Am. Chem. Soc. 2009, 131, 6372–6373. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Yamauchi, Y.; Hill, J.P.; Miyazawa, K.i.; Ariga, K. Fullerene Crystals with Bimodal Pore Architectures Consisting of Macropores and Mesopores. J. Am. Chem. Soc. 2013, 135, 586–589. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Xu, T.; Lu, X. Three-Dimensional “Star of David”-Shaped Fullerene (C60) Microstructures: Controlled Synthesis, Photoluminescence, and Photoelectrochemical Properties. ACS Appl. Electron. Mater. 2020, 2, 2010–2016. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Liu, D.; Yao, M.; Hou, Y.; Yu, S.; Cui, T.; Li, D.; Zou, G.; Iwasiewicz, A.; et al. Synthesis of Thin, Rectangular C60 Nanorods Using M-Xylene as a Shape Controller. Adv. Mater. 2006, 18, 1883–1888. [Google Scholar] [CrossRef]
- David, W.I.F.; Ibberson, R.M.; Matthewman, J.C.; Prassides, K.; Dennis, T.J.S.; Hare, J.P.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Crystal Structure and Bonding of Ordered C60. Nature 1991, 353, 147–149. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Yu, S.; Yao, M.; Liu, D.; Hou, Y.; Cui, T.; Zou, G.; Sundqvist, B.; You, H.; et al. Highly Enhanced Luminescence from Single-Crystalline C60·1m-Xylene Nanorods. Chem. Mater. 2006, 18, 4190–4194. [Google Scholar] [CrossRef]
- Rana, M.; Reddy, R.B.; Rath, B.B.; Gautam, U.K. C60-Mediated Molecular Shape Sorting: Separation and Purification of Geometrical Isomers. Angew. Chem. Int. Ed. 2014, 53, 13523–13527. [Google Scholar] [CrossRef]
- Henn, D.E.; Williams, W.G.; Gibbons, D.J. Crystallographic Data for an Orthorhombic Form of Rubrene. J. Appl. Crystallogr. 1971, 4, 256. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Wang, C.; Yang, F.; Ren, X.; Zhang, X.; Dong, H.; Hu, W. Organic Crystalline Materials in Flexible Electronics. Chem. Soc. Rev. 2019, 48, 1492–1530. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, X.; Guan, Y.; Wang, Y.; Jin, F.; Guan, R.; Liu, F.; Chen, M.; Tian, Y.; Yang, S. From Cubes to Dice: Solvent-Regulated Morphology Engineering of Endohedral Fullerene Microcrystals with Anomalous Photoluminescence Enhancement. Angew. Chem. Int. Ed. 2019, 58, 11350–11354. [Google Scholar] [CrossRef]
- Slanina, Z.; Rudziński, J.M.; Togasi, M.; Ōsawa, E. Quantum-Chemically Supported Vibrational Analysis of Giant Molecules: The C60 and C70 Clusters. J. Mol. Struct. Teochem 1989, 202, 169–176. [Google Scholar] [CrossRef]
- Xie, Y.; Boggs, J.E. The Computed Force Constants and Vibrational Spectra of Toluene. J. Comput. Chem. 1986, 7, 158–164. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Scott, D.W. The Thermodynamics and Molecular Structure of Benzene and Its Methyl Derivatives1. J. Am. Chem. Soc. 1943, 65, 803–829. [Google Scholar] [CrossRef]
- Zhang, K.K.; Tan, K.; Zou, C.; Wikberg, M.; McNeil, L.E.; Mhaisalkar, S.G.; Kloc, C. Control of Charge Mobility in Single-Crystal Rubrene through Surface Chemistry. Org. Electron. 2010, 11, 1928–1934. [Google Scholar] [CrossRef]
- Weinberg-Wolf, J.R.; McNeil, L.E.; Liu, S.; Kloc, C. Evidence of Low Intermolecular Coupling in Rubrene Single Crystals by Raman Scattering. J. Phys. Condens. Matter 2007, 19, 276204. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, S.; Saha, A.; Bao, L.; Lu, X.; Guldi, D.M. Understanding Charge-Transfer Characteristics in Crystalline Nanosheets of Fullerene/(Metallo)Porphyrin Cocrystals. J. Am. Chem. Soc. 2017, 139, 10578–10584. [Google Scholar] [CrossRef]
- Wakahara, T.; Sathish, M.; Miyazawa, K.i.; Hu, C.; Tateyama, Y.; Nemoto, Y.; Sasaki, T.; Ito, O. Preparation and Optical Properties of Fullerene/Ferrocene Hybrid Hexagonal Nanosheets and Large-Scale Production of Fullerene Hexagonal Nanosheets. J. Am. Chem. Soc. 2009, 131, 9940–9944. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Yambem, S.D.; Moore, E.G.; Namdas, E.B.; Pandey, A.K. Singlet Fission and Triplet Exciton Dynamics in Rubrene/Fullerene Heterojunctions: Implications for Electroluminescence. Adv. Electron. Mater. 2015, 1, 1500229. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, M.; Lu, X. Facile Method toward Hierarchical Fullerene Architectures with Enhanced Hydrophobicity and Photoluminescence. ACS Appl. Mater. Interfaces 2015, 7, 20285–20291. [Google Scholar] [CrossRef]
- Yan, D.; Delori, A.; Lloyd, G.O.; Friščić, T.; Day, G.M.; Jones, W.; Lu, J.; Wei, M.; Evans, D.G.; Duan, X. A Cocrystal Strategy to Tune the Luminescent Properties of Stilbene-Type Organic Solid-State Materials. Angew. Chem. Int. Ed. 2011, 50, 12483–12486. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal Engineering: A Collaborative Strategy toward Functional Materials. Adv. Mater. 2019, 31, 1902328. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Pang, J.; Ni, S.; Zhang, G.; Xu, L.; Dang, L.; Li, M.-D. Insight into Intermolecular Charge Transfer Determined by Two Packing Mode Cocrystals. J. Phys. Chem. C 2020, 124, 17744–17751. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, Y.; Liao, L.; Hu, W. Competition between Arene-Perfluoroarene and Charge-Transfer Interactions in Organic Light-Harvesting Systems. Angew. Chem. Int. Ed. 2017, 56, 10352–10356. [Google Scholar] [CrossRef]
- Pinto, R.M. Photocurrent Generation in Bulk Vs Bilayer Devices: Quantum Treatment of Model Rubrene/7,7,8,8-Tetracyanoquinodimethane Heterojunctions for Organic Solar Cells. J. Phys. Chem. C 2014, 118, 2287–2297. [Google Scholar] [CrossRef]
- Pinto, R.M.; Maçôas, E.M.S.; Alves, H. Enhanced Conductivity and Photoresponse at a Rubrene Single-Crystal–Pcbm Film Interface. J. Mater. Chem. C 2014, 2, 3639–3644. [Google Scholar] [CrossRef]
- Saran, R.; Curry, R.J. Solution Processable 1d Fullerene C60 Crystals for Visible Spectrum Photodetectors. Small 2018, 14, 1703624. [Google Scholar] [CrossRef]
- Wang, M.; Xing, C.; Cao, K.; Zhang, L.; Liu, J.; Meng, L. Template-Directed Synthesis of Pyrite (FeS2) Nanorod Arrays with an Enhanced Photoresponse. J. Mater. Chem. A 2014, 2, 9496–9505. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Pan, H.; Zhang, X.; Zhang, Y.; Zhang, X.; Jie, J. Large-Area Aligned Growth of Single-Crystalline Organic Nanowire Arrays for High-Performance Photodetectors. Nanotechnology 2013, 24, 355201. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Y.; Wang, H.; Samorì, P. Organic Photodetectors Based on Supramolecular Nanostructures. SmartMat 2020, 1, e1009. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Ma, D. Fast Response Organic Photodetectors with High Detectivity Based on Rubrene and C60. Org. Electron. 2013, 14, 3019–3023. [Google Scholar] [CrossRef]
- Jin, J.; Wu, S.; Ma, Y.; Dong, C.; Wang, W.; Liu, X.; Xu, H.; Long, G.; Zhang, M.; Zhang, J.; et al. Nucleation Control-Triggering Cocrystal Polymorphism of Charge-Transfer Complexes Differing in Physical and Electronic Properties. ACS Appl. Mater. Interfaces 2020, 12, 19718–19726. [Google Scholar] [CrossRef] [PubMed]
- Marjanović, N.; Singh, T.B.; Dennler, G.; Günes, S.; Neugebauer, H.; Sariciftci, N.S.; Schwödiauer, R.; Bauer, S. Photoresponse of Organic Field-Effect Transistors Based on Conjugated Polymer/Fullerene Blends. Org. Electron. 2006, 7, 188–194. [Google Scholar] [CrossRef]
- Wang, G.; Chen, D.; Zhang, H.; Zhang, J.Z.; Li, J. Tunable Photocurrent Spectrum in Well-Oriented Zinc Oxide Nanorod Arrays with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2008, 112, 8850–8855. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, N.; Yu, P.; Guo, K.; Lu, X. Rubrene-Directed Structural Transformation of Fullerene (C60) Microsheets to Nanorod Arrays with Enhanced Photoelectrochemical Properties. Nanomaterials 2022, 12, 954. https://doi.org/10.3390/nano12060954
Chen N, Yu P, Guo K, Lu X. Rubrene-Directed Structural Transformation of Fullerene (C60) Microsheets to Nanorod Arrays with Enhanced Photoelectrochemical Properties. Nanomaterials. 2022; 12(6):954. https://doi.org/10.3390/nano12060954
Chicago/Turabian StyleChen, Ning, Pengwei Yu, Kun Guo, and Xing Lu. 2022. "Rubrene-Directed Structural Transformation of Fullerene (C60) Microsheets to Nanorod Arrays with Enhanced Photoelectrochemical Properties" Nanomaterials 12, no. 6: 954. https://doi.org/10.3390/nano12060954
APA StyleChen, N., Yu, P., Guo, K., & Lu, X. (2022). Rubrene-Directed Structural Transformation of Fullerene (C60) Microsheets to Nanorod Arrays with Enhanced Photoelectrochemical Properties. Nanomaterials, 12(6), 954. https://doi.org/10.3390/nano12060954