The Construct CoSe2 on Carbon Nanosheets as High Sensitivity Catalysts for Electro-Catalytic Oxidation of Glucose
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Co-Se Carbon Nanomaterials
2.3. Characterization
2.4. Electrochemical Measurement
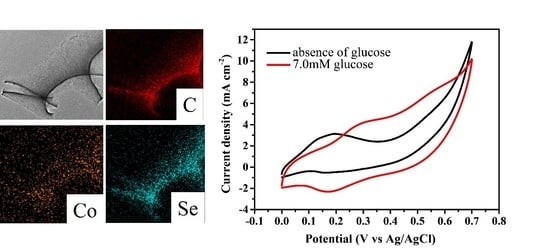
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Chen, H.; Wang, Y.; Ying, Y. Recent advances in the rational synthesis and sensing applications of metal-organic framework biocomposites. Coord. Chem. Rev. 2019, 387, 60–78. [Google Scholar] [CrossRef]
- Lee, W.-C.; Kim, K.-B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.-S.; Shim, Y.-B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosen. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bukkitgar, S.D.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Reddy, C.V.; Sadhu, V.; Naveen, S. Electrochemical sensors and biosensors based on graphene functionalized with metal oxide nanostructures for healthcare applications. Chemistryselect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Brinda, K.N.; Achar, G.; Malecki, J.G.; Budagumpi, S.; Nagaraju, D.H.; Suvina, V.; Balakrishna, R.G. Glucose oxidase mimicking half–sandwich nickel (II) complexes of coumarin substituted N–heterocyclic carbenes as novel molecular electrocatalysts for ultrasensitive and selective determination of glucose. Biosens. Bioelectron. 2019, 134, 24–28. [Google Scholar] [CrossRef]
- Hao, X.; Jia, J.; Chang, Y.; Jia, M.; Wen, Z. Monodisperse copper selenide nanoparticles for ultrasensitive and selective non-enzymatic glucose biosensor. Electrochim. Acta 2019, 327, 135020. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, Y.; Wang, M.; Zhong, N.; Kang, Z.; Asao, N.; Jiang, W.J.; Chen, Q. Composition-dependent morphology of bi-and trimetallic phosphides: Construction of amorphous Pd–Cu–Ni–P nanoparticles as a selective and versatile Catalyst. ACS Appl. Mater. Interfaces 2017, 9, 34804–34811. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Dinesh, B.; Rethinasabapathy, M.; Hwang, S.-K.; Jin, C.-S.; Huh, Y.S.; Han, Y.-K. Hexagonal Co3O4 anchored reduced graphene oxide sheets for high-performance supercapacitors and non-enzymatic glucose sensing. J. Mater. Chem. A 2018, 6, 14367–14379. [Google Scholar] [CrossRef]
- Brownlee, B.J.; Bahari, M.; Harb, J.N.; Claussen, J.C.; Iverson, B.D. Electrochemical glucose sensors enhanced by methyl viologen and vertically aligned carbon nanotube channels. ACS Appl. Mat. Interfaces 2018, 10, 28351–28360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Qian, L.; Zhang, K.; Yuan, S.; Xiao, J.; Wang, S. Hierarchical porous Ni/NiO core–shells with superior conductivity for electrochemical pseudo-capacitors and glucose sensors. J. Mater. Chem. A 2015, 3, 10519–10525. [Google Scholar] [CrossRef]
- Soomro, R.A.; Ibupoto, Z.H.; Sirajuddin; Abro, M.I.; Willander, M. Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sens. Actuators B Chem. 2015, 209, 966–974. [Google Scholar] [CrossRef]
- Chen, F.; Li, J.-H.; Chi, Y.-C.; Dan, Z.-H.; Qin, F.-X. Synthesis of Novel Pd Nanosponges for Non-Enzymatic Glucose Sensor. J. Nanosci. Nanotechnol. 2020, 20, 7333–7341. [Google Scholar] [CrossRef]
- De Sa, M.H.; Brandao, L. Non-enzymatic direct glucose fuel cells (DGFC): A novel principle towards autonomous electrochemical biosensors. Int. J. Hydrogen Energy 2020, 45, 29749–29762. [Google Scholar] [CrossRef]
- Savk, A.; Aydin, H.; Cellat, K.; Sen, F. A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt-Ni nanocomposite. J. Mol. Liq. 2020, 300, 112355. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Teng, J.; Wong, W.-L.; Qiu, B. In situ deposition of MOF-74 (Cu) nanosheet arrays onto carbon cloth to fabricate a sensitive and selective electrocatalytic biosensor and its application for the determination of glucose in human serum. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, R.; He, F.; Hu, W.; Cao, X.; Jia, J.; Lu, W.; Sun, X. A comparative study of electrocatalytic oxidation of glucose on conductive Ni-MOF nanosheet arrays with different ligands. New J. Chem. 2020, 44, 17849–17853. [Google Scholar] [CrossRef]
- Khazraei, A.; Tarlani, A.; Eslami-Moghadam, M.; Muzart, J. New Bi2MoO6 nano-shapes toward ultrasensitive enzymeless glucose tracing: Synergetic effect of the Bi-Mo association. Talanta 2021, 221, 121560. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosen. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhao, H.; Bi, S.; Xu, Y.; Lu, Y. Ultrasensitive and highly selective sandpaper-supported copper framework for non-enzymatic glucose sensor. Electrochim. Acta 2017, 248, 281–291. [Google Scholar] [CrossRef]
- Xu, H.; Han, F.; Xia, C.; Wang, S.; Zhuiykov, S.; Zheng, G. Spinel sub-stoichiometric CuxCoyO4 nano-wire framework thin-film electrode for enhanced electrochemical non-enzymatic sensing of glucose. Electrochim. Acta 2020, 331, 135295. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Tian, X.; Wang, X.; Yu, Y.; Owusu, K.A.; He, L.; Mai, L. Porous nickel–iron selenide nanosheets as highly efficient electrocatalysts for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 19386–19392. [Google Scholar] [CrossRef] [PubMed]
- Swesi, A.T.; Masud, J.; Nath, M. Nickel selenide as a high-efficiency catalyst for oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1771–1782. [Google Scholar] [CrossRef]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 9351–9355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jia, J.; Chang, Y.; Jia, M.; Wen, Z. CoSe2 nanocrystals embedded into carbon support as coralline-like catalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 22787–22795. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Chang, Y. The Construct CoSe2 on Carbon Nanosheets as High Sensitivity Catalysts for Electro-Catalytic Oxidation of Glucose. Nanomaterials 2022, 12, 572. https://doi.org/10.3390/nano12030572
Wang D, Chang Y. The Construct CoSe2 on Carbon Nanosheets as High Sensitivity Catalysts for Electro-Catalytic Oxidation of Glucose. Nanomaterials. 2022; 12(3):572. https://doi.org/10.3390/nano12030572
Chicago/Turabian StyleWang, Di, and Ying Chang. 2022. "The Construct CoSe2 on Carbon Nanosheets as High Sensitivity Catalysts for Electro-Catalytic Oxidation of Glucose" Nanomaterials 12, no. 3: 572. https://doi.org/10.3390/nano12030572
APA StyleWang, D., & Chang, Y. (2022). The Construct CoSe2 on Carbon Nanosheets as High Sensitivity Catalysts for Electro-Catalytic Oxidation of Glucose. Nanomaterials, 12(3), 572. https://doi.org/10.3390/nano12030572