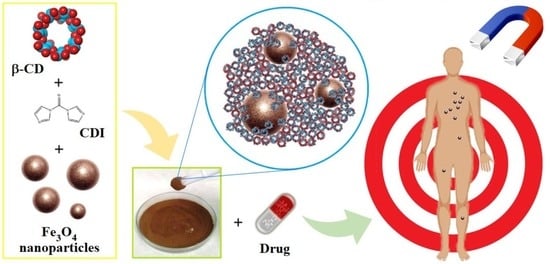
Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Magnetite Nanoparticles
2.2. Synthesis of Magnetic Nanosponges
2.3. Preparation of Fe3O4-Decorated NS
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Thermogravimetric Analysis (TGA)
2.6. CHNS Analysis
2.7. X-ray Powder Diffraction Studies (XRD)
2.8. FESEM Analysis
2.9. HRTEM Analysis
2.10. Magnetisation Curves
2.11. Stability Study
2.12. Loading of Doxo in Magnetic Dextrin-Based Nanosponges
2.13. Quantification of the Content of Doxo
2.14. Release Studies
3. Results and Discussion
3.1. Physicochemical Characterisation
3.2. Encapsulation of Doxorubicin and Release Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duchêne, D. Cyclodextrins and Their Inclusion Complexes. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 1–18. [Google Scholar]
- Shi, L.; Zhou, J.; Guo, J.; Gladden, I.; Kong, L. Starch inclusion complex for the encapsulation and controlled release of bioactive guest compounds. Carbohydr. Polym. 2021, 274, 118596. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of cyclodextrin nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Caldera, F.; Dianzani, C.; Argenziano, M.; Barrera, G.; Cavalli, R. Glutathione Bioresponsive Cyclodextrin Nanosponges. ChemPlusChem 2016, 81, 439–443. [Google Scholar] [CrossRef]
- Momin, M.M.; Zaheer, Z.; Zainuddin, R.; Sangshetti, J.N. Extended release delivery of erlotinib glutathione nanosponge for targeting lung cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1064–1075. [Google Scholar] [CrossRef] [Green Version]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813–35829. [Google Scholar] [CrossRef]
- Fontana, R.M.; Milano, N.; Barbara, L.; Di Vincenzo, A.; Gallo, G.; Meo, P.L. Cyclodextrin-Calixarene Nanosponges as Potential Platforms for pH-Dependent Delivery of Tetracycline. ChemistrySelect 2019, 4, 9743–9747. [Google Scholar] [CrossRef]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2019, 7, 91–114. [Google Scholar] [CrossRef]
- Ngandeu Neubi, G.M.; Opoku-Damoah, Y.; Gu, X.; Han, Y.; Zhou, J.; Ding, Y. Bio-inspired drug delivery systems: An emerging platform for targeted cancer therapy. Biomater. Sci. 2018, 6, 958–973. [Google Scholar] [CrossRef]
- Ye, X.; Yang, D. Recent Advances in Biological Strategies for Targeted Drug Delivery. Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Chen, D.; Shang, P.; Yin, D.C. A review of magnet systems for targeted drug delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Hui, D.; Hong, R. Progress in magnetic Fe3O4 nanomaterials in magnetic resonance imaging. Nanotechnol. Rev. 2020, 9, 1265–1283. [Google Scholar] [CrossRef]
- Bai, C.; Hu, P.; Liu, N.; Feng, G.; Liu, D.; Chen, Y.; Ma, M.; Gu, N.; Zhang, Y. Synthesis of Ultrasmall Fe3O4 Nanoparticles as T1–T2 Dual-Modal Magnetic Resonance Imaging Contrast Agents in Rabbit Hepatic Tumors. ACS Appl. Nano Mater. 2020, 3, 3585–3595. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.; Maier-Hauff, K. Magnetic Nanoparticles for Intracranial Thermotherapy. J. Nanosci. Nanotechnol. 2007, 7, 4604–4606. [Google Scholar] [CrossRef]
- Gavilan, H.; Avugadda, S.K.; Fernandez-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Siemieniec, J.; Kafarski, P.; Plucinski, P. Hydrophosphonylation of nanoparticle Schiff bases as a mean for preparation of aminophosphonate-functionalized nanoparticles. Molecules 2013, 18, 8473–8484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cengelli, F.; Grzyb, A.J.; Montoro, A.; Hofmann, H.; Hanessian, S.; Juillerat-Jeanneret, L. Surface-Functionalized Ultrasmall Superparamagnetic Nanoparticles as Magnetic Delivery Vectors for Camptothecin. ChemMedChem 2009, 4, 988–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Zhang, K.; Li, S.; Wu, J.; Pham-Huy, C.; Diao, X.; Xiao, D.; He, H. Superparamagnetic Fe3O4 nanoparticles: Synthesis by a solvothermal process and functionalization for a magnetic targeted curcumin delivery system. New J. Chem. 2016, 40, 4480–4491. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Miri, R.; Salmanpour, M.; Khalighian, N.; Sotoudeh, S.; Erfani, N. Preparation and assessment of chitosan-coated superparamagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res. Pharm. Sci. 2013, 8, 25–33. [Google Scholar] [PubMed]
- Aguilera, G.; Berry, C.C.; West, R.M.; Gonzalez-Monterrubio, E.; Angulo-Molina, A.; Arias-Carrión, Ó.; Méndez-Rojas, M.Á. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood–brain barrier. Nanoscale Adv. 2019, 1, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Saikia, C.; Hussain, A.; Ramteke, A.; Sharma, H.K.; Maji, T.K. Carboxymethyl starch-chitosan-coated iron oxide magnetic nanoparticles for controlled delivery of isoniazid. J. Microencapsul. 2015, 32, 29–39. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Jedrzak, A.; Szutkowski, K.; Grzeskowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, A.P.F.; Caminhas, L.D.; Ardisson, J.D.; Paniago, R.; Cortes, M.E.; Sinisterra, R.D. Magnetic nanoparticles coated with cyclodextrins and citrate for irinotecan delivery. Carbohydr. Polym. 2017, 163, 1–9. [Google Scholar] [CrossRef]
- Cai, K.; Li, J.; Luo, Z.; Hu, Y.; Hou, Y.; Ding, X. β-Cyclodextrin conjugated magnetic nanoparticles for diazepam removal from blood. Chem. Commun. 2011, 47, 7719–7721. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.-H. Magnetic Nanoparticles Grafted with Cyclodextrin for Hydrophobic Drug Delivery. Chem. Mater. 2007, 19, 6345–6349. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic beta-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. Int. J. Mol. Sci. 2020, 21, 4079. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. A synthetic guide toward the tailored production of magnetic iron oxide nanoparticles. Bol. Soc. Esp. Ceram. Vidr. 2021, 60, 29–40. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic Materials and Systems: Domain Structure Visualization and Other Characterization Techniques for the Application in the Materials Science and Biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Nistico, R.; Bianco Prevot, A.; Magnacca, G.; Canone, L.; Garcia-Ballesteros, S.; Arques, A. Sustainable Magnetic Materials (from Chitosan and Municipal Biowaste) for the Removal of Diclofenac from Water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Mártire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Nistico, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef]
- Sung, H.W.F.; Rudowicz, C. Physics behind the magnetic hysteresis loop—a survey of misconceptions in magnetism literature. J. Magn. Magn. Mater. 2003, 260, 250–260. [Google Scholar] [CrossRef]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based Nanosponges for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing Limited: Cambridge, UK, 2015; pp. 63–86.







| Sample | DMF (mL) | Fe3O4 Nanoparticles (g) | β-CD (g) | LC (g) | CDI (g) |
|---|---|---|---|---|---|
| β5% | 20 | 0.031 | 3.333 | - | 3.810 |
| β10% | 20 | 0.061 | 3.333 | - | 3.810 |
| LC5% | 20 | 0.031 | - | 3.333 | 3.810 |
| LC10% | 20 | 0.061 | - | 3.333 | 3.810 |
| Sample | N (%) | C (%) | H (%) | S (%) |
|---|---|---|---|---|
| β5% | 0.33 | 37.65 | 5.52 | 0.00 |
| β10% | 0.52 | 37.93 | 5.39 | 0.00 |
| LC5% | 0.29 | 37.88 | 5.49 | 0.00 |
| LC10% | 0.30 | 37.16 | 5.36 | 0.00 |
| Samples | Saturation Magnetisation, Ms (emu/g of Material) | Saturation Magnetisation, Ms (emu/g of Iron Oxide) | Magnetic Remanence, Mr (emu/g of Material) | Intrinsic Coercivity, Hic (Oe) | Ref. |
|---|---|---|---|---|---|
| Magnetite | - | 64 | 1.0 | 10 | [37] |
| β5% | 2 | 43 | 0.1 | 12 | Present study |
| β10% | 4 | 48 | <0.1 | 14 | Present study |
| LC5% | 19 | 312 | 3.1 | 120 | Present study |
| LC10% | 4 | 48 | 0.1 | 11 | Present study |
| Doxo-Loaded NSs | Loading Capacity (%) | Encapsulation Efficiency (%) |
|---|---|---|
| β5% | 2.88 | 5.30 |
| β10% | 2.31 | 4.25 |
| LC5% | 3.32 | 6.11 |
| LC10% | 2.60 | 4.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldera, F.; Nisticò, R.; Magnacca, G.; Matencio, A.; Khazaei Monfared, Y.; Trotta, F. Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery. Nanomaterials 2022, 12, 754. https://doi.org/10.3390/nano12050754
Caldera F, Nisticò R, Magnacca G, Matencio A, Khazaei Monfared Y, Trotta F. Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery. Nanomaterials. 2022; 12(5):754. https://doi.org/10.3390/nano12050754
Chicago/Turabian StyleCaldera, Fabrizio, Roberto Nisticò, Giuliana Magnacca, Adrián Matencio, Yousef Khazaei Monfared, and Francesco Trotta. 2022. "Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery" Nanomaterials 12, no. 5: 754. https://doi.org/10.3390/nano12050754
APA StyleCaldera, F., Nisticò, R., Magnacca, G., Matencio, A., Khazaei Monfared, Y., & Trotta, F. (2022). Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery. Nanomaterials, 12(5), 754. https://doi.org/10.3390/nano12050754